Abstract
Background
Pain might be associated with cognitive impairment in humans. However, the characterization of such effects in a preclinical model and the investigation of the underlying mechanisms remain largely to be determined. We therefore sought to establish a system to determine the effect of pain on cognitive function in mice.
Methods
Complete Freund's adjuvant (CFA) was injected in the hindpaw of 5–8-month-old wild-type and interleukin-6 knockout mice. Learning and memory function, and the levels of interleukin-6 and postsynaptic density (PSD)-95 in the cortex and hippocampus of mice were assessed.
Results
We found that the CFA injection induced pain in the mice at 3 and 7 days after injection and decreased the freezing time [30.1 (16.5) seconds versus 56.8 (28.1) seconds, P = 0.023] in the tone test, which assesses the hippocampus-independent learning and memory function, but not in a context test of Fear Conditioning System [15.8 (6.7) seconds versus 18.6 (8.8) seconds, P = 0.622], which assesses the hippocampus-dependent learning and memory function, at 3 days after injection. Consistently, the CFA injection increased interleukin-6 [248% (11.6) versus 100% (7.9), P < 0.0001] and decreased the PSD-95 [40% (10.0) versus 100% (20.3), P < 0.0001] level in the cortex, but not hippocampus [95%(8.6) versus 100%(9.3), P = 0.634], in the mice. The CFA injection induced neither reduction in the cortex PSD-95 levels nor cognitive impairment in the interleukin-6 knockout mice.
Conclusion
These results suggest that pain induced by CFA injection might increase interleukin-6 levels and decrease PSD-95 levels in the cortex, but not hippocampus of mice, leading to hippocampus-independent cognitive impairment in mice. These findings call for further investigation to determine the role of pain in cognitive function.
Introduction
There are about 116 million Americans living with pain according to the Institute of Medicine.1 Clinical studies have reported that pain could be associated with neurological disorders, including cognitive impairment.2–7 A recent study has suggested that there is an association between pain and neurocognitive dysfunction.8 Specifically, patients with generalized pain and neuropathic pain, but not localized pain, fail certain cognitive tests more easily.8 Therefore, it is important to establish a preclinical model to investigate the effects of pain on cognitive function and the underlying mechanisms.
It has been reported that neuroinflammation, including elevation of the levels of proinflammatory cytokine, may lead to cognitive dysfunction [reviewed in9]. Specifically, proinflammatory cytokine interlukin-6 (IL-6) has been shown to be associated with cognitive dysfunction and mild cognitive impairment in medical and surgical patients.10 IL-6 has also been shown to be involved in learning and memory function in rodents.11–16 Postsynaptic density 95 (PSD-95) is a postsynaptic marker.17,18 The decreases in PSD-95 levels are associated with a reduction in synapse number or synaptic loss, and impairment of learning and memory.16,19–22 The effects of pain on the levels of IL-6 and PSD-95 in brain tissues of mice, however, have not been assessed.
Finally, the context and tone test of the Fear Conditioning System (FCS) can be used to assess hippocampus-dependent and hippocampus-independent learning and memory, respectively.23–26 Our previous studies have shown that sleep disturbance can induce neuroinflammation and decrease the freezing time in the context test, but not tone test, of the FCS, which suggests that sleep disturbance may selectively induce neuroinflammation in the hippocampus and induce hippocampus-dependent learning and memory impairment in mice.14 However, whether pain can cause hippocampus-independent or hippocampus-dependent changes in neurochemistry and neurobehavioral function remains unknown.
We established a preclinical system in mice to assess whether pain could induce hippocampus-dependent (assessing by context test of FCS) or hippocampus-independent (assessing by tone test of FCS) learning and memory impairment in mice and alter the levels of IL-6 and PSD-95 in the cortex or hippocampus of mice.
Methods
Animals
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, Massachusetts. Wild-type (WT) C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) and IL-6 knockout (KO) mice (B6.129S2-Il6tm1Kopf/J, the same genetic background C57BL/6J) (5–8 months old, The Jackson Laboratory) were randomly assigned to a complete Freund's adjuvant (CFA) injection or control group. A previous study has shown that the pain threshold in IL-6 KO mice is similar to that in WT mice.27 Mice were housed in a controlled environment (20 – 22°C; 12 hour light: dark on a reversed light cycle) for one week before the studies. The maintenance and handling of mice were consistent with the guidelines of the National Institute of Health, and all efforts were made to minimize the number of animals studied.
Injection of complete Freund's adjuvant
Each mouse was briefly anesthetized with isoflurane (1.4% for 5 minutes). The mouse then received 20 µl of CFA (Sigma, St. Louis, MO) or saline in the bottom of the right hindpaw as described in detail in a previous study.28 CFA consists of heat-killed Mycobacterium tuberculosis in 85% paraffin oil and 15% mannide monoleate and is often used to induce pain (chemically induced) in rodents in pain research.28
Pain threshold test
Pain threshold was determined by using nylon von Frey filaments as described in a previous study.29 Mice were placed on a wire mesh platform in clear cylindrical plastic enclosures 8-centimeter (cm) diameter and 10 cm in height. After 20 minutes on the platform, filaments were applied (bending force range from 0.008 to 26 gram; North Coast Medical, Inc., San Jose, CA) on the wound edge of the CFA-injected hindpaw for approximately 5 seconds with a 10-second interval between each stimulation. Withdrawal of the hindpaw from the floor was scored as a positive response. When no response was obtained, the next stiffer filament in the series was applied to the same paw. Each monofilament was applied to the hindpaw 5 times. The hindpaw withdrawal threshold (the pain threshold) was obtained as the force (in gram) at which foot withdrawal occurred for at least 3 of the five stimulations.
Fear Conditioning System
The FCS is a behavioral procedure designed to assess associative learning and memory. FCS has been often used to detect learning and memory impairment induced by anesthesia alone, anesthesia with surgery, and sleep disturbance.14 We performed FCS studies as described in our previous studies and other studies.30,31 Specifically, the mice were exposed for training in the FCS (Stoelting Co., Wood Dale, IL) 24 hours after CFA injection. Each mouse was allowed to explore the chamber for 180 seconds before presentation of a 2-Hz pulsating tone (80 dB, 1,500 Hz) that persisted for 60 seconds. The tone was followed immediately by a mild foot shock (0.8 mA for 0.5 seconds). Context (no tone period) and tone (tone period) learning and memory were probed 3, 7 and 14 days after the training in sequence. Mice in the same group were given the context test first, then a tone test one hour later. For the context test, each mouse (from either the control group or the CFA injection group) was allowed to stay in the chamber for 180 seconds, followed by another 180-second period without a tone, and finally 30 seconds for recovery. For the tone test, each mouse (from either the control group or the CFA injection group) was allowed to stay in the chamber for 180 seconds, followed by another 180-second period with a tone, and finally 30 seconds for recovery. Learning and memory were assessed by measuring the amount of time the mouse demonstrated “freezing behavior,” defined as a completely immobile posture except for respiratory efforts, during the test period (the second 180-second period), which was analyzed by Any-Maze (Stoelting Co., Wood Dale, IL). The first 180-second period allowed mice to adjust to the environment before counting freezing time in the second 180-second period.
Brain tissue harvest and protein level quantification
Different groups of mice from both the control condition and the pain condition were used for biochemistry studies. Three, 7 and 14 days after injection of CFA, mice were killed by decapitation (for Western blot analysis). The brain cortex (whole cerebral cortex) and hippocampus were harvested separately. Using a mouse brain map, the brain cortex and hippocampus were visually identified by the observers. For Western blot analysis, the harvested brain cortex or hippocampus was homogenized on ice using immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 µg ml−1 aprotinin, 1 µg ml−1 leupeptin, 1 µg ml−1 pepstatin A). The lysates were collected, centrifuged at 12,000 rpm for 10 minutes, and quantified for total proteins with a Bicinchoninic Acid protein assay kit (Pierce, Iselin, NJ).
Western blot analysis
IL-6 antibody (1:1,000 dilution, Abcam, Cambridge, MA) was used to recognize IL-6 (24 kDa). PSD-95 antibody (1:1,000, Cell Signaling, Danvers, MA) was used to detect PSD-95 (95 kDa). Antibody anti-β-Actin (1:10,000, Sigma, St. Louis, MO) was used to detect β-Actin (42 kDa). Western blot quantification was performed as described by Xie et al.32 Briefly, signal intensity was analyzed using a Bio-Rad (Hercules, CA) image program (Quantity One). We quantified Western blots in two steps, first, we used β-Actin levels to normalize (e.g., determine the ratio of IL-6 to β-Actin amount) protein levels and control for loading differences in the total protein amount. Second, we presented protein level changes in mice in the CFA injection group as a percentage of those in the control group. One-hundred percent of protein level changes refer to control levels for the purpose of comparison of experimental conditions.
Statistics
Data are expressed as mean and standard deviation (SD). The number of samples varied from 9–10. Samples were normally distributed (tested by Shapiro-Wilk test, data not shown). A two-tailed Student t-test (the Student's t-test with unequal variances or Welch's method) was used to compare differences in freezing time, IL-6 levels, and PSD-95 levels between the CFA injection and control groups. Mice at 3, 7, and 14 days after injection were separate groups of mice, thus one-way Analysis of Variance (ANOVA) was used to analyze the levels of IL-6 and PSD-95 between control group and the CFA injection group at 3, 7 and 14 days after injection. Post hoc analyses were performed if the main effect was found to be statistically significant. The respective p-value was Bonferroni-adjusted by the number of comparisons made, 4 pairs for IL-6 levels and 4 pairs for PSD-95 levels. Specifically, we used multiple two-group Student t-tests with the P-values Bonferroni correction to compare the difference in IL-6 and PSD-95 levels between the control and the CFA injection groups at 3, 7 or 14 days after injection. P values less than 0.05 (*) and 0.01 (**) were considered statistically significant. Significance testing was two tailed, and Prism 6 software (La Jolla, CA, USA) was used to analyze the data.
Results
CFA injection induced pain in mice
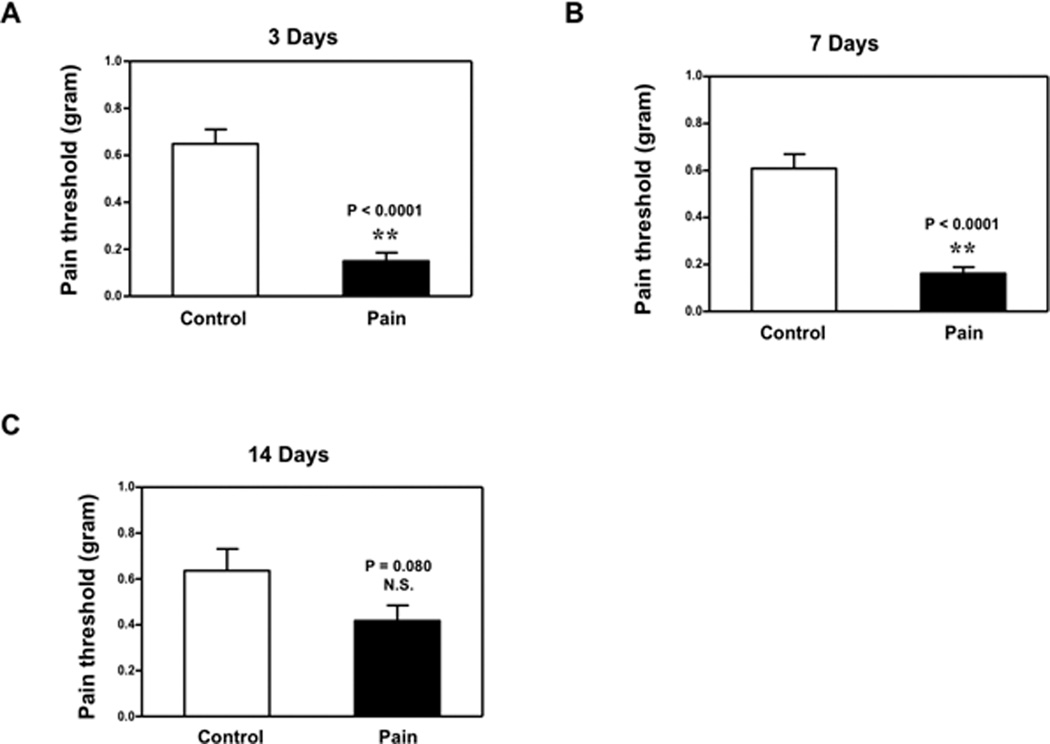
We first established a preclinical system of producing pain in mice by injection of CFA into the righthind paw. The injection of CFA reduced the pain threshold at 3 days after injection (Figure 1A): 0.15 (0.11) gram versus 0.65 (0.19) gram, P < 0.0001, and 7 days after injection (Figure 1B): 0.16 (0.08) gram versus 0.61 (0.19) gram, P < 0.0001. CFA injection also reduced the pain threshold at 14 days after injection; however, the difference did not reach significance (Figure 1C): 0.42 (0.21) versus 0.64 (0.31) gram, P = 0.077. These data showed that CFA injection in mice paws could cause pain, which demonstrated that we were able to establish a preclinical model of pain for the current experiments.
Figure 1. Injection of complete Freund’s adjuvant (CFA) produced chemically-induced pain in mice.
A. Injection of 20 ul complete Freund's adjuvant (CFA) in the bottom of the right hindpaw of mice (black bar) reduces pain threshold compared to injection of 20 ul saline (white bar) at 3 days after injection. B. Injection of 20 ul CFA in the bottom of the right hindpaw of mice (black bar) also reduces pain threshold compared to injection of 20 ul saline (white bar) at 7 days after injection. C. Injection of 20 ul CFA in the bottom of right hindpaw of mice (black bar) does not significantly reduce pain threshold compared to injection of 20 ul saline (white bar) at 14 days after injection. N = 10.
CFA injection-induced pain caused transient cognitive impairment in mice
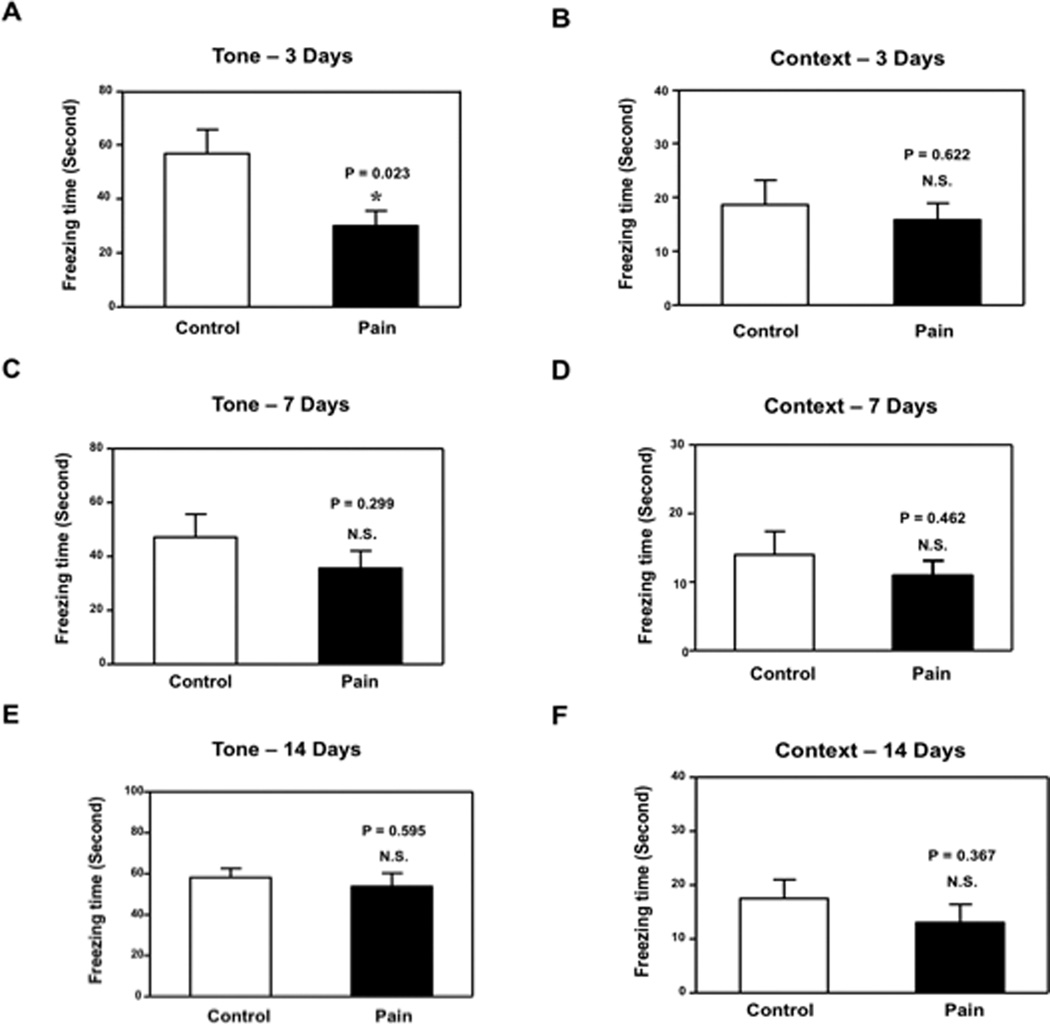
Given that we established a preclinical model of pain, we asked whether CFA injection-induced pain in mice might cause cognitive impairment. We determined the effects of CFA injection-induced pain on the function of learning and memory in the FCS. Different groups of mice were used in the FCT studies at 3, 7 and 14 days after injection. FCS studies showed that CFA injection-induced pain (Figure 2A, black bar) led to decreases in freezing time in the FCS tone test as compared to the control condition (Figure 2A, white bar) at 3 days after injection: 30.1 (16.5) seconds versus 56.8 (28.1) seconds, P = 0.023. Next, we found that CFA injection-induced pain did not decrease freezing time in the FCS context test compared to the control condition at 3 days after injection: black bar versus white bar, P = 0.622. (Figure 2B). We found that CFA injection-induced pain did not significantly change the freezing time in the FCS tone or context test compared to the control condition at 7 (Figure 2C, P = 0.299; Figure 2D, P = 0.462) and 14 days (Figure 2E, P = 0.595; Figure 2F, P = 0.367) after injection in the mice. These results suggest that the CFA injection-induced pain may induce a transient impairment of learning and memory function in mice at 3, but not 7 and 14, days after injection. Moreover, CFA injection-induced pain might selectively impair hippocampus-independent learning and memory function (detected by tone test in FCS) in mice.
Figure 2. Complete Freund's adjuvant (CFA) injection induced pain-impaired learning and memory in mice.
A. Complete Freund's adjuvant (CFA) injection decreased freezing time in the tone test of the Fear Conditioning System (FCS) as compared to the control condition at 3 days after injection B. CFA injection did not decrease freezing time in the context test of the FCS as compared to the control condition at 3 days after injection. C. CFA injection did not decrease freezing time in the tone test of the FCS as compared to the control condition at 7 days after injection. D. CFA injection did not decrease freezing time in the context test of the FCS as compared to the control condition at 7 days after injection. E. CFA injection did not decrease freezing time in the tone test of the FCS as compared to the control condition at 14 days after injection. F. CFA injection did not decrease freezing time in the context test of the FCS as compared to the control condition at 14 days after injection. N = 10.
CFA injection-induced pain caused transient increase in IL-6 levels in the cortex, but not hippocampus, of mice
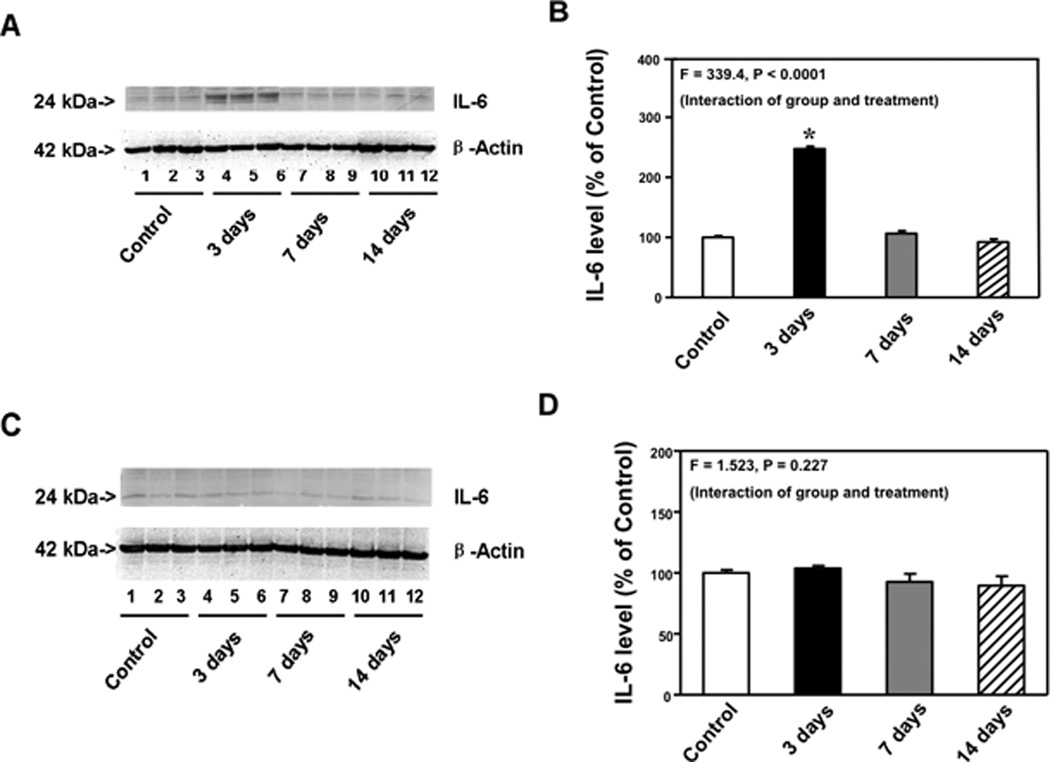
Given that IL-6 is associated with cognitive function in rodents11–16 and that CFA injection-induced pain caused cognitive impairment in the mice, we next asked whether CFA injection-induced pain could increase IL-6 levels in brain tissues of mice. Immunoblotting of IL-6 demonstrated that CFA injection-induced pain increased IL-6 levels in cortexes as compared to the control condition at 3, but not 7 and 14, days after injection (Figure 3A). There was no significant difference in brain β-Actin levels between the control condition and CFA injection condition (Figure 3A). Western blot quantification, based on the ratio of IL-6 to β-Actin illustrated that there was a significant difference in IL-6 levels in the cortexes between the control condition and CFA injection condition at 3, 7 and 14, days after injection (Figure 3B, one-way ANOVA, F = 339.4, P < 0.0001). Post hoc test showed that CFA injection-induced pain increased IL-6 levels in cortexes as compared to the control condition at 3 [248% (11.7) versus 100% (7.9), P < 0.05], but not 7 [107% (13.1) versus 100% (7.9), P > 0.05] and 14 [92.4% (14.7) versus 100% (7.9), P > 0.05] days after injection. CFA injection-induced pain did not increase IL-6 levels in mice hippocampus at 3, 7 and 14 days after (Figure3C and 3D). These data suggest that CFA injection-induced pain might cause a transient increase in IL-6 levels in mouse brain tissues. Moreover, CFA injection-induced pain specifically increased IL-6 levels in cortexes but not in hippocampus, which was consistent with the behavioral findings that CFA injection-induced pain selectively impaired hippocampus-independent learning and memory function.
Figure 3. Complete Freund's adjuvant (CFA) injection-induced pain increased IL-6 levels in the cortexes of mice.
A. Complete Freund's adjuvant (CFA) injection increased interleukin (IL)-6 levels in cortexes as compared to control condition in Western blot analysis at 3, but not 7 and 14, days after injection. There was no significant difference in the amounts of β-Actin in the cortexes after CFA injection or the control condition. B. Western blot quantification shows that CFA injection increased IL-6 levels in the cortexes as compared to the control condition at 3, but not 7 and 14, days after injection. C. CFA injection did not increase IL-6 levels in the hippocampus as compared to control condition in Western blot analysis at 3, 7 and 14 days after injection. There was no significant difference in the amounts of β-Actin in the hippocampus after CFA injection or the control condition. D. Western blot quantification shows that CFA injection did not increase IL-6 levels in the hippocampus as compared to the control condition at 3, 7 and 14 days after injection. N = 9.
CFA injection-induced pain caused a transient decrease in postsynaptic density 95 levels in the cortex, but not hippocampus, of mice
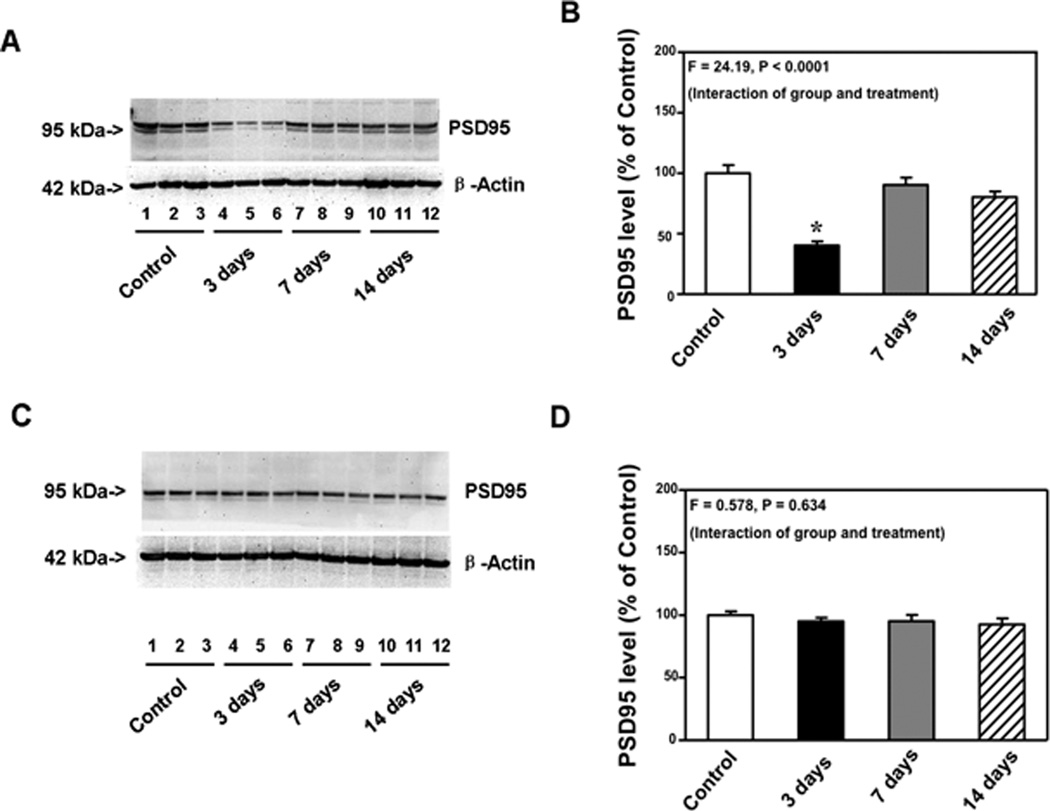
Postsynaptic marker PSD-95 has been shown to be associated with synapse number or synaptic loss. A previous study has shown that anesthetic-induced reduction in PSD-95 levels can be inhibited by IL-6 antibody in mouse primary neurons.16 Given that CFA injection-induced pain increased IL-6 levels in the cortexes of mice, we then asked whether CFA injection-induced pain could reduce PSD-95 levels in brain tissues. PSD-95 immunoblotting showed that CFA injection-induced pain led to a visible reduction in the levels of bands representing PSD-95 in cortexes at 3, but not 7 and 14 days after injection (Figure 4A). There was no significant difference in β-Actin levels between the control and pain condition in mice (Figure 4A). Western blot quantification was based on the ratio of PSD-95 to β-Actin. One-way ANOVA showed that there was a significant difference in the PSD-95 levels of cortexes after the control condition, and pain condition at 3, 7 and 14 days after injection (Figure 4B, F = 24.19, P < 0.0001). Post hoc test showed that CFA injection-induced pain decreased PSD-95 levels in the cortexes as compared to the control condition at 3 days after injection: 40% (10.0) versus 100% (20.4), P < 0.05, but not 7 days [90% (18.0) versus 100% (20.4), P > 0.05] and 14 days [80% (13.7) versus 100% (20.4), P > 0.05] after injection. CFA injection-induced pain in the mice did not decrease PSD-95 levels in hippocampus at 3, 7 and 14 days after injection (Figure4C and 4D). These data suggested that CFA injection-induced pain might cause a transient decrease in the synapse number in mouse brain tissues. Moreover, CFA injection-induced pain could specifically decrease synapse number in cortexes but not in mice hippocampus, which was consistent with the findings that CFA injection-induced pain selectively increased IL-6 levels in cortexes (but not mice hippocampus) and impaired the hippocampus-independent learning and memory function.
Figure 4. Complete Freund's adjuvant (CFA) injection-induced pain decreased PSD-95 levels in the cortexes of mice.
A. Complete Freund's adjuvant (CFA) injection decreased postsynaptic density 95 (PSD-95) levels in cortexes as compared to the control condition in Western blot analysis at 3, but not 7 and 14, days after injection. There was no significant difference in the amount of β-Actin in the cortexes after CFA injection or the control condition. B. Western blot quantification shows that CFA injection decreased PSD-95 levels in the cortexes as compared to the control condition at 3, but not 7 and 14, days after injection. C. CFA injection did not decrease PSD-95 levels in the hippocampus as compared to the control condition in Western blot analysis at 3, 7 and 14 days after injection. There was no significant difference in the amount of β-Actin in the hippocampus after CFA injection or the control condition. D. Western blot quantification shows that CFA injection did not decrease PSD-95 levels in the hippocampus as compared to the control condition at 3, 7 and 14 days after injection. N = 9.
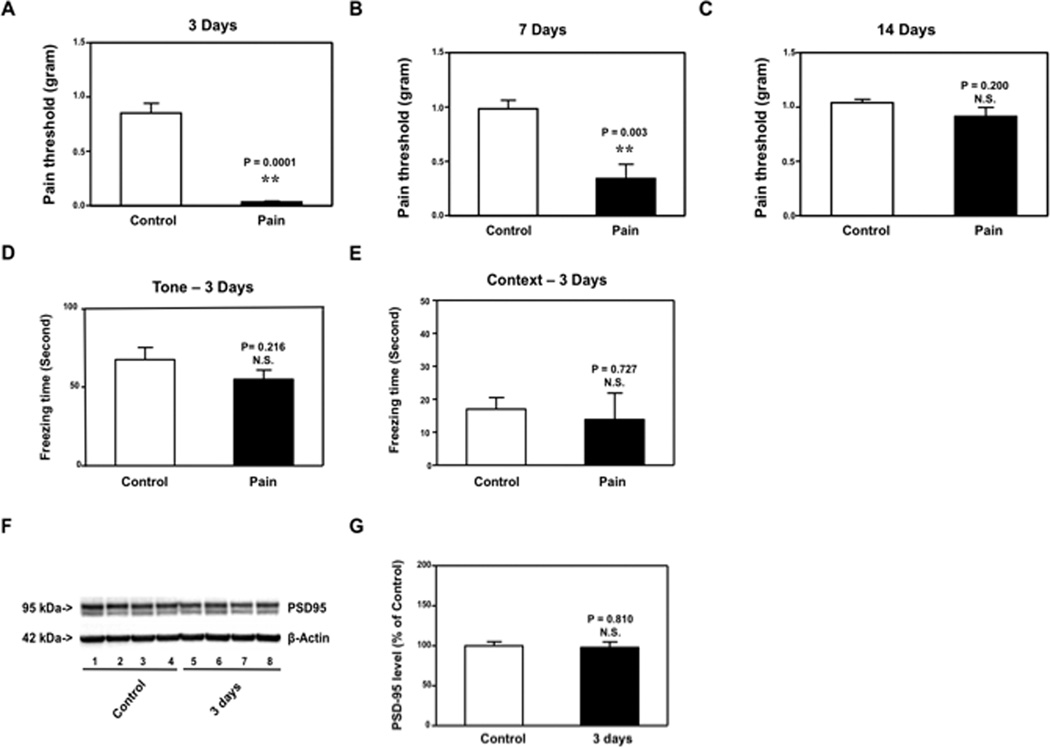
CFA injection-induced pain caused neither cognitive impairment nor reduction in PSD-95 levels in IL-6 KO mice
Given that CFA injection-induced pain in mice increased IL-6 levels, decreased PSD-95 levels in the cortexes of mice, and caused cognitive impairment, we next asked whether the effects of CFA injection-induced pain on brain PSD-95 levels as well as learning and memory function were dependent on IL-6. We used IL-6 KO mice in the studies and found that CFA injection reduced the pain threshold in IL-6 KO mice at 3 [0.032 (0.02) gram versus 0.851 (0.24) gram, P = 0.0001] and 7 [0.340 (0.33) gram versus 0.983 (0.21) gram, P = 0.003], but not 14 [0.913 (0.20) gram versus 1.04 (0.08) gram, P = 0.200] days after injection (Figure 5A, 5B and 5C). We then found that CFA injection-induced pain did not reduce PSD-95 levels in cortexes at 3 days after injection (Figure5D and 5E). Finally, we found that CFA injection-induced pain did not reduce freezing time in the FCS tone and context test at 3 days after injection (Figure5F and 5G). These results demonstrated that the decreases in PSD-95 levels in cortexes and impairment of learning and memory resulting from CFA injection-induced pain might be dependent on elevation of IL-6 levels. Collectively, these findings suggested that CFA injection-induced pain increased IL-6 levels in cortexes, but not hippocampus, which reduced PSD-95 levels in cortexes, but not hippocampus, leading to hippocampus-independent, but not hippocampus-dependent, cognitive impairment.
Figure 5. Complete Freund's adjuvant (CFA) injection-induced pain induced neither impairment of learning and memory nor reduction in mice cortex PSD-95 levels in IL-6 knockout (KO) mice.
Complete Freund's adjuvant (CFA) injection decreases pain threshold in interleukin (IL)-6 KO mice at 3 (A) and 7 (B), but not 14 (C), days after injection. CFA injection did not reduce freezing time in tone test (D) or context test (E) of the Fear Conditioning System (FCS) as compared to control condition at 3 days after injection in IL-6 KO mice. F. In IL-6 KO mice, CFA injection did not reduce postsynaptic density 95 (PSD-95) levels in the cortexes of IL-6 KO mice as compared to control condition in the Western blot analysis at 3 days after injection. There was no significant difference in the amount of β-Actin in the cortexes after CFA injection or the control condition in IL-6 KO mice. G. Western blot quantification shows that CFA injection did not decrease PSD-95 levels in the cortexes as compared to the control condition at 3 days after injection in IL-6 KO mice.. N = 9 for the biochemistry studies, N = 10 for the behavioral studies.
Discussion
Clinical studies have suggested that pain may be associated with cognitive impairment in humans;2–8 however, assessing the effects of pain on cognitive function and the underlying mechanisms remain largely undetermined. We therefore established a preclinical model in mice to determine the effects of CFA injection-induced pain on learning and memory function and the levels of IL-6 and PSD-95.
We first demonstrated that the CFA injection in the hindpaw of mice caused pain at 3, 7, and 14 days after injection, although the difference did not reach significant levels at 14 days after injection (Figure 1). We then were able to show that CFA injection-induced pain caused a transient impairment of learning and memory in mice at 3, but not 7 and 14, days after injection (Figure 2).
FCS is among the most commonly used behavioral tests to detect cognitive impairment.14,30,31,33–35 The FCS context and tone test can be used to assess hippocampus-dependent and hippocampus-independent learning and memory function.23–26 In the present experiments, CFA injection-induced pain only reduced freezing time in the FCS tone test, but not the context test (Figure 2). These findings suggest that CFA injection-induced pain might selectively cause hippocampus-independent cognitive impairment. Radial maze and specific paradigms of contextual fear conditioning are traditional learning tasks used to assess hippocampus-dependent cognitive function.36,37,38 These behavioral tasks are not available to us at the present time. However, we may develop these tasks to further prove that CFA injection-induced pain can selectively cause hippocampus-independent cognitive impairment in our future studies.
CFA injection-induced pain also increased IL-6 levels and reduced PSD-95 levels in the cortexes of the mice (Figure 3 and 4). Both IL-6 elevation and PSD-95 reduction are associated with cognitive impairment.10–22,36,37 Therefore, these results suggested that CFA injection-induced pain might likely cause cognitive impairment through the elevation of IL-6 levels and the reduction of PSD-95 levels. Interestingly, CFA injection-induced pain only increased IL-6 levels and decreased PSD-95 levels in cortexes, but not hippocampus. CFA injection-induced pain also selectively caused hippocampus-independent, but not hippocampus-dependent, cognitive impairment. These results suggest that there may be associations between CFA-induced pain, changes in brain IL-6 and PSD95 levels, and cognitive function. Moreover, CFA injection-induced pain might cause cognitive impairment in mice through an IL-6-dependent and PSD-95-associated mechanism. However, other mechanisms, e.g., CFA-induced neuroinflammation, may also be involved in these changes, which need to be investigated further.
CFA injection in IL-6 KO mice caused neither cognitive impairment nor reduction of PSD-95 levels at 3 days after injection (Figure 5). These data showed that the effects of CFA injection-induced pain on PSD-95 and cognitive function were dependent on the elevation of IL-6, and further suggested that the CFA injection-induced pain might lead to cognitive impairment via elevation of IL-6 and reduction of PSD-95 in the brain. It is also possible that inflammatory pain and cognitive impairment after CFA injection may not go through the same set of inflammatory cytokines because IL-6 knockout mice still have pain but without cognitive impairment.
Elevation of proinflammatory cytokine IL-6 is part of neuroinflammation, and neuroinflammation may cause cognitive dysfunction [reviewed in9]. IL-6 has been suggested to contribute to cognitive dysfunction and mild cognitive impairment in medical and surgical patients.10 In animal studies, IL-6 has also been shown to cause cognitive impairment in rodents.11–16 IL-6 has been reported to contribute to neuropathic pain development. Specifically, intrathecal infusion of IL-6 induces mechanoallodynia in nerve-intact mice and thermal hyperalgesia in nerve-injured rats.38 KO of IL-6 may contribute to the development of tight ligation and transection of L5 spinal nerve-induced mechanoallodynia but not thermal allodynia in mice.27 The current studies have found that IL-6 may be involved in neuroinflammatory pain-induced cognitive impairment. Moreover, our recent study has shown that surgical incision-induced pain may cause cognitive impairment via reduction of the synaptic N-Methyl-D-aspartate receptor 2B.39 Taken together, these data suggest the association of pain, neuroinflammation, synaptic function and cognitive function. It is therefore important to further investigate the role of IL-6 in pain, neuroinflammation, synaptic function and cognitive impairment and elucidate the underlying mechanisms. Such studies would facilitate future mechanistic investigation of pain-associated cognitive impairment.
CFA injection-induced pain lasted at least 7 days after injection. However, the injection of CFA induced hippocampus-independent cognitive impairment and changes in IL-6 and PSD-95 levels in the cortex at 3, but not 7 and 14, days after injection (Figure 3 and 4). The exact reason underlying these findings is unknown. However, these findings suggest that there could be some tolerance effects after CFA injection-induced pain, which would limit the duration of pain-induced cognitive impairment. Further studies to test this hypothesis are warranted.
It is unclear why CFA injection-induced pain only increased IL-6 levels and reduced PSD-95 levels in the cortex, but not hippocampus, and caused hippocampus-independent cognitive impairment. A previous study has also shown that sleep disturbance selectively induces neuroinflammation in hippocampus and hippocampus-dependent cognitive impairment in mice.14 Therefore, it is conceivable that different physical stimulation or disturbance may affect different brain regions and the associated behavioral changes. Moreover, a recent study demonstrated that pain could induce a neural “signature,” a brain-wide pattern of excitation and inhibition, in specific brain regions, e.g., cortex.40 The findings from our current studies suggest that CFA injection-induced pain in mice may induce a brain region-specific change in neuroinflammation, synapse number and neurobehavioral deficits.
A study by Shu et al. has shown that injection of formalin in the hindpaw of mice induced neurotoxicity, e.g., increases in the levels of caspase-3 positive cells in brain tissues,41 which is consistent with our current findings that injection of CFA in the hindpaw increased IL-6 levels and reduced PSD-95 levels. Chemically induced pain may not cause long-term cognitive impairment because the injection of formalin41 or CFA (the findings from the current study) did not cause cognitive impairment at 14 and 7 days after injection, respectively. Interestingly, injection of formalin did not increase the levels of tumor necrosis factor-α and IL-1β, but the injection of CFA increased IL-6 levels in brain tissues of mice. The underlying mechanisms of this difference needs further investigation.
Lunardi et al. reported that anesthetics (nitrous oxide and isoflurane) can impair synaptogenesis, including the reduction in synapse number and disturbances in ultrastructural properties of developing synapses in young rats.42 These data suggest that anesthetics may induce cognitive impairment in young rodents via impairment of synaptogenesis. Our current studies showed that pain might also affect synapse function, e.g., reduction in PSD-95 levels. Anesthetics have been shown to enhance pain-induced neurotoxicity (e.g., caspase-3 activation).41 Therefore, it is conceivable that anesthetics may also enhance pain-induced synapse dysfunction, e.g., reduction in PSD-95 levels, leading to increased cognitive impairment. Future studies to determine whether pain and anesthetics can enhance each other’s effects in causing synapse dysfunction and cognitive impairment are warranted.
Our study has several limitations. First, we only assessed the effects of CFA injection-induced pain on IL-6 and PSD-95 levels in the hippocampus and cortex. Future studies should determine whether CFA injection-induced pain could also increase IL-6 levels and reduce PSD-95 levels in other brain regions, e.g., the amygdala. Nevertheless, the current findings suggest that CFA injection-induced pain increased IL-6 levels in the cortex, but not hippocampus of mice which then reduced PSD-95 levels in the cortex, leading to hippocampus-independent cognitive impairment (Figures 2, 3, 4). Second, we did not assess whether analgesia, e.g., eutectic mixture of lidocaine and prilocaine cream (2.5% lidocaine and 2.5% prilocaine), could rescue the changes in IL-6, PSD-95 and cognitive function after CFA injection-induced pain. This was mainly because eutectic mixture of lidocaine and prilocaine cream might not be effective in treating CFA injection-induced pain, because of the inflammatory tissues. Moreover, we did not want pain medicine, e.g., morphine, to influence CFA injection-induced neuroinflammation, reduction in synapse number and neurobehavioral deficits. The injection of CFA has been well shown to induce pain in rodents.43–45 Therefore, the current findings that CFA injection-induced cognitive impairment, increased IL-6 levels and reduced PSD-95 levels support our hypothesis that chemically induced pain can induce neuroinflammation, reduction in synapse number and neurobehavioral deficits.
In conclusion, in this proof of concept study, we established a preclinical model of pain in mice and found that the injection of CFA could cause pain. CFA injection-induced pain might increase proinflammatory cytokine IL-6 levels and decrease PSD-95 levels in cortexes, but not in hippocampus. CFA injection-induced pain also impaired hippocampus-independent learning and memory. Finally, KO of IL-6 (in IL-6 KO mice) prevented the effects of CFA injection-induced pain on PSD-95 levels and cognitive impairment. These findings should encourage studies further studies to determine the role of pain in cognitive function decline. Any new studies should include assessment of underlying mechanism(s) by which chemically induced pain induces brain region-specific neuroinflammation and reduction in synapse number, and the associated neurobehavioral deficits.
Acknowledgments
Funding: This research was supported by R21AG038994, R01 GM088801 and R01 AG041274 from National Institutes of Health, Bethesda, Maryland, Investigator-initiated Research grant from Alzheimer’s Association, Chicago, Illinois, and Cure Alzheimer’s Fund, Wellesley, Massachusetts to Zhongcong Xie.
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES:
Name: Longqiu Yang, MD, PhD
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Longqiu Yang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Xin Xin, MD
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Xin Xin has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Jie Zhang, MD
Contribution: This author helped write the manuscript
Attestation: Jie Zhang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Lei Zhang, MD PhD
Contribution: This author helped write the manuscript
Attestation: Lei Zhang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Yuanlin Dong, MD
Contribution: This author helped write the manuscript
Attestation: Yuanlin Dong has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Yiying Zhang, MD
Contribution: This author helped write the manuscript
Attestation: Yiying Zhang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Jianren Mao, MD, PhD
Contribution: This author helped design the study and write the manuscript
Attestation: Jianren Mao has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Zhongcong Xie, MD, PhD
Contribution: This author helped design the study and write the manuscript
Attestation: Zhongcong Xie has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Recuse Note: Jianren Mao is the Section Editor for Pain and Analgesic Mechanisms for the Journal. This manuscript was handled by Dr. Steven L. Shafer, Editor-in-Chief, and Dr. Mao was not involved in any way with the editorial process or decision.
Contributor Information
Longqiu Yang, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts (Current Affiliation: Department of Anesthesiology, Zhengzhou Central Hospital, Nanjing, China).
Xin Xin, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts (Current Affiliation: Department of Anaesthesia, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P. R. China).
Jie Zhang, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts (Current Affiliation: Department of Anaesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, P. R. China).
Lei Zhang, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts (Current Affiliation: Department of Anaesthesia, the Ninth Hospital of Shanghai, School of Medicine, Shanghai Jiao Tong University, Shanghai, P. R. China).
Yuanlin Dong, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts.
Yiying Zhang, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts.
Jianren Mao, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts.
Zhongcong Xie, Geriatric Anaesthesia Research Unit, Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital of Harvard Medical School, Charlestown, Massachusetts.
References
- 1.Institute of Medicine (US) Committee on Advancing Pain Research Care, and Education: Relieving Pain in America. A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Dick BD, Pillai Riddell R. Cognitive and school functioning in children and adolescents with chronic pain: a critical review. Pain Res Manag. 2010;15:238–244. doi: 10.1155/2010/354812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10:131–149. doi: 10.1023/a:1009020914358. [DOI] [PubMed] [Google Scholar]
- 4.McCracken LM, Iverson GL. Predicting complaints of impaired cognitive functioning in patients with chronic pain. J Pain Symptom Manage. 2001;21:392–396. doi: 10.1016/s0885-3924(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil. 2005;86:1147–1154. doi: 10.1016/j.apmr.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Schnurr RF, MacDonald MR. Memory complaints in chronic pain. Clin J Pain. 1995;11:103–111. doi: 10.1097/00002508-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Landro NI, Fors EA, Vapenstad LL, Holthe O, Stiles TC, Borchgrevink PC. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? Pain. 2013;154:972–977. doi: 10.1016/j.pain.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: The role and consequences. Neuroscience research. 2013 doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2010 doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- 11.Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, Iosue S, Sala M. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res. 2004;153:423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–355. doi: 10.1016/j.nbd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective Anesthesia-induced Neuroinflammation in Developing Mouse Brain and Cognitive Impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, Xie Z. Sevoflurane Anesthesia in Pregnant Mice Induces Neurotoxicity in Fetal and Offspring Mice. Anesthesiology. 2013;118:516–526. doi: 10.1097/ALN.0b013e3182834d5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ, Bu G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010;30:17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hongpaisan J, Sun MK, Alkon DL. PKC {varepsilon} Activation Prevents Synaptic Loss, Abeta Elevation, and Cognitive Deficits in Alzheimer's Disease Transgenic Mice. J Neurosci. 2011;31:630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 21.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 22.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 23.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, Silva AJ. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol. 2010;20:1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain. 1998;78:115–121. doi: 10.1016/S0304-3959(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 28.Kassuya CA, Silvestre AA, Rehder VL, Calixto JB. Anti-allodynic and anti-oedematogenic properties of the extract and lignans from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain. Eur J Pharmacol. 2003;478:145–153. doi: 10.1016/j.ejphar.2003.08.079. [DOI] [PubMed] [Google Scholar]
- 29.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 30.Saab BJ, Maclean AJ, Kanisek M, Zurek AA, Martin LJ, Roder JC, Orser BA. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113:1061–1071. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- 31.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patanella AK, Zinno M, Quaranta D, Nociti V, Frisullo G, Gainotti G, Tonali PA, Batocchi AP, Marra C. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res. 2010;88:1106–1112. doi: 10.1002/jnr.22276. [DOI] [PubMed] [Google Scholar]
- 37.Schuitemaker A, Dik MG, Veerhuis R, Scheltens P, Schoonenboom NS, Hack CE, Blankenstein MA, Jonker C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–1889. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 38.DeLeo JA, Colburn RW, Nichols M, Malhotra A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1996;16:695–700. doi: 10.1089/jir.1996.16.695. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Xin X, Dong Y, Zhang Y, Yu B, Mao J, Xie Z. Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17737–17748. doi: 10.1523/JNEUROSCI.2049-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu Y, Zhou Z, Wan Y, Sanders RD, Li M, Pac-Soo CK, Maze M, Ma D. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–750. doi: 10.1016/j.nbd.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotoxicity research. 2010;17:179–188. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasser A, Bjerrum OJ, Heegaard AM, Moller AT, Larsen M, Dalboge LS, Dupont E, Jensen TS, Moller LB. Impaired behavioural pain responses in hph-1 mice with inherited deficiency in GTP cyclohydrolase 1 in models of inflammatory pain. Mol Pain. 2013;9:5. doi: 10.1186/1744-8069-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolstad AM, Rodriguiz RM, Kim CJ, Hale LP. Effect of pain management on immunization efficacy in mice. J Am Assoc Lab Anim Sci. 2012;51:448–457. [PMC free article] [PubMed] [Google Scholar]
- 45.Schiene K, De Vry J, Tzschentke TM. Antinociceptive and antihyperalgesic effects of tapentadol in animal models of inflammatory pain. J Pharmacol Exp Ther. 2011;339:537–544. doi: 10.1124/jpet.111.181263. [DOI] [PubMed] [Google Scholar]