Abstract
Semiochemicals are volatile compounds that communicate specific meaning between individuals, and elicit specific behavioral and/or physiological responses mediated by highly sensitive and highly specific olfactory pathways. Recent work suggests that semiochemicals can activate multiple olfactory pathways at once, but the degree to which parallel pathways activated by the same semiochemical interact, and what the behavioral consequences of such interactions are, remains a topic of debate. Here we approached this question behaviorally, investigating whether rats could be trained to avoid carbon disulfide (CS2) via taste-potentiated odor aversion, and asking whether any such learning would have an impact on the rats’ subsequent use of CS2 as a semiochemical cue (i.e., in a socially transmitted food preference paradigm). The results show that CS2-mediated food preference learning is unimpaired by aversions conditioned to CS2, a result indicating that canonical and semiochemical pathways for the processing of CS2 function in a largely independent manner.
Keywords: carbon disulfide, pheromones, semiochemicals, olfaction
Animals communicate with each other using a wide variety of physical and chemical signals. The meaning of such signals—their significance to the conspecific being communicated with—is often flexible, and can be influenced by experience, a prime example being the malleability of human language. The meaning of semiochemicals (the class of chemical signals that includes pheromones), on the other hand, is thought to be highly stable. Semiochemicals, by definition, are organism-emitted cues that carry intrinsic value and elicit specific behavioral or physiological responses that are mediated by highly sensitive and highly specific olfactory pathways in the main and accessory olfactory systems (Hu et al., 2007; Leinders-Zufall et al., 2007; Leinders-Zufall et al., 2000; Liberles & Buck, 2006).
Recent work demonstrates, however, that semiochemicals also activate canonical olfactory pathways—that is, G protein-coupled olfactory sensory neurons (OSNs) in the main olfactory epithelium that are responsible for odor perception (D. Y. Lin, Zhang, Block, & Katz, 2005; W. Lin, Arellano, Slotnick, & Restrepo, 2004; Luo, Fee, & Katz, 2003; Munger et al., 2010; Xu et al., 2005). This fact suggests that semiochemical perception may be subject to experience-dependent plasticity typically associated with processing in the canonical olfactory pathway (Mandairon & Linster, 2009). The degree to which the multiple pathways activated by the same semiochemical interact, and what the behavioral consequences of such interactions are, remains a topic of debate (Munger, Leinders-Zufall, & Zufall, 2009). For instance, can an intrinsically positive semiochemical be rendered aversive, and if so, does this learned aversion impede its semiochemical function?
In the present work, we assessed the independence of rats’ canonical (olfactory) and semiochemical perception of carbon disulfide (CS2)—a semiochemical that communicates consumability of a food from one conspecific to another (B. Galef, Mason, Preti, & Bean, 1988; Munger et al., 2010). CS2 is a dominant and highly volatile component of rodent breath that, when smelled in combination with a food odor, increases preference of the observer for that food (Fortis-Santiago, Rodwin, Neseliler, Piette, & Katz, 2010; B. Galef et al., 1988; B. G. Galef & Wigmore, 1983; Munger et al., 2010). CS2-mediated social transmission of food preference from demonstrator to observer can occur with exposure as brief as 5 minutes (Burne, Johnston, Wilkinson, & Kendrick, 2010) and is highly robust. For example, preferences are effectively transmitted even when the food is aversive (B. Galef et al., 1988), and even when the demonstrator is prone, ill, or unconscious (Burne et al., 2010; B. G. Galef, Jr., Wigmore, & Kennett, 1983). Thus, preference induction by CS2 apparently involves little evaluation of current context, suggesting that the meaning of CS2 may be independent of experience—i.e., stable and unmodifiable. This semiochemical function of CS2 appears to begin with transduction via a specialized subset of OSNs expressing guanylyl cyclase D (GC-D) receptors. However, CS2 also appears to activate canonical OSNs in addition to GC-D OSNs (Munger et al., 2010).
In order to test the hypothesis that CS2 is processed by two separate neural pathways, we first attempted to condition an aversion to CS2, using a taste-potentiated odor aversion paradigm; subsequently, we tested the same animals’ ability to use CS2 as a semiochemical in learning socially transmitted food preferences. The results demonstrate that rats indeed learn aversions to CS2, just as they learn aversions to commonly used non-pheromonal odors. CS2-induced food preference learning, however, is unimpeded by this newfound aversion. These data imply the existence of multiple independent olfactory processing systems, supported either by multiple transduction pathways or contextual variables that emphasize activity in one activated circuit over another.
Materials and Methods
Subjects
We used male Long-Evans rats (n=43, www.criver.com), weighing between 250 and 325 g at the start of experiments. All animals were individually housed and kept on a 12/12 hour light/dark cycle. All experiments were conducted during the light cycle, and complied with the Brandeis University Institutional Animal Care and Use Committee guidelines.
Behavioral procedures
Taste-potentiated odor aversion (TPOA)
Over the entire course of the TPOA protocol, access to fluids was restricted to the amount consumed during experimental sessions described below.
Each session began with animals placed into cages inside sound- and light-attenuating chambers. The first 30 minutes of each session was acclimatization, after which a lick spout, attached to a 50 ml conical tube, was introduced and animals were allowed to drink freely for seven minutes. Consumption was measured by weighing bottles before and after sessions.
A piece of filter paper saturated with an odor stimulus was secured near the base of the lick spout; both filter paper and lick spout were surrounded by a narrow, cylindrical piece of plastic such that animals had to stick their nose in to gain access to fluid. The presence of the plastic sleeve ensured the rat’s exposure to the odor while drinking, but made it impossible for the rat to reach the odor source itself.
The experimental protocol consisted of the following sequence of sessions, occurring on consecutive days: 1) Habituation (3–4 days); 2) Training (1 day); 3) Testing (3 days). During habituation sessions, bottles contained de-ionized water (dH2O) and no odor stimuli were presented (i.e., the filter paper was dry). Habituation sessions were repeated until water intake reached a stable level. During the training session, bottles contained 0.15% sodium saccharin solution (in dH2O), and the saccharin was accompanied by filter paper wetted with either CS2 or benzaldehyde (5 µl pure odorant; www.sigmaaldrich.com); immediately after the training session, animals were briefly anesthetized with isofluorane and injected subcutaneously with 0.6 M lithium chloride (LiCl; 2% of body weight) (Nachman & Ashe, 1973). One animal was excluded from analysis because it drank less than 1 ml during training.
The first subsequent testing session probed for an odor aversion; the second for a taste aversion; and the third (only performed in a subset of rats) for aversion to the testing environment. During the first testing session, bottles contained dH2O, and the filter paper was wetted with same odor stimulus that had been present in the training session; on the second testing day, bottles contained 0.15% saccharin solution and were presented with dry filter paper; on the third testing day, rats were presented with dH2O, again with dry filter paper. Relative aversion was calculated by:
consumption on testing day / consumption on training day
For one animal, taste aversion could not be assessed due to a leaking bottle.
One hour after each session, animals had 30 minute access to 10 ml water in their testing environment.
Social Transmission of Food Preference (STFP)
Over the entire course of the STFP protocol, access to food was restricted to the amount consumed during experimental sessions described below.
The STFP paradigm consisted of the following sessions, occuring on consecutive days: 1) Habituation (4–5 days); 2) Training (1 day); and 3) Testing (1 day). During habituation sessions, observer and demonstrator animals were familiarized with the interaction procedure, and with consuming a powdered version of regular chow from ramekins. Before the training session, demonstrator animals received one of two novel flavored foods A and B—powdered regular chow mixed either with cinnamon (1%, www.mccormick.com) or cocoa (2%, www.hersheys.com) powder; note that this differentiates STFP from the “poisoned partner effect” (Hishimura, 2000; Iraola & Alonso, 1995), wherein it is the subject rat that consumes the CS prior to the interaction. Observer and demonstrator animals were then placed together in an opaque plastic cage and allowed to interact for 30 minutes.
For the preference testing session, observer animals were placed in novel opaque cages for 1 hour and offered 8 g each of flavored foods A and B (placed side by side in a shallow plastic tub, ramekins separated by a pane of clear Plexiglas). Identity and spatial location of the demonstrated flavor—the flavor that had been eaten by the demonstrator rat prior to the training interaction—was counterbalanced across animals. Consumption was measured by weighing each powdered food sample before and after sessions. Relative preference for was calculated by:
demonstrated food consumed / total food consumed
For two animals, preference could not be assessed due to spilled food.
Results
Experiment I
In the taste-potentiated odor aversion (TPOA) paradigm, animals learn an aversion to the odor component of a taste-odor compound (the conditioned stimulus) associated with gastric distress (the unconditioned stimulus). The well-known robustness of TPOA (Palmerino, Rusiniak, & Garcia, 1980; Rusiniak, Hankins, Garcia, & Brett, 1979; Slotnick, Westbrook, & Darling, 1997) makes it an excellent tool with which to challenge the stability of the CS2 representation.
In a single 7 minute training session, animals drank a saccharin solution near the source of orthonasally presented CS2 (n=17) or, alternatively, of an orthonasally presented non-semiochemical odorant (benzaldehyde, n=12). Directly after this session, gastric malaise was induced by injection of lithium chloride. On subsequent days, aversion to the odor stimulus was tested by monitoring consumption of plain water in the context of the odor stimulus, and aversion to saccharin was tested by monitoring consumption of saccharin in the absence of odor stimuli (see Figure 2).
Figure 2.
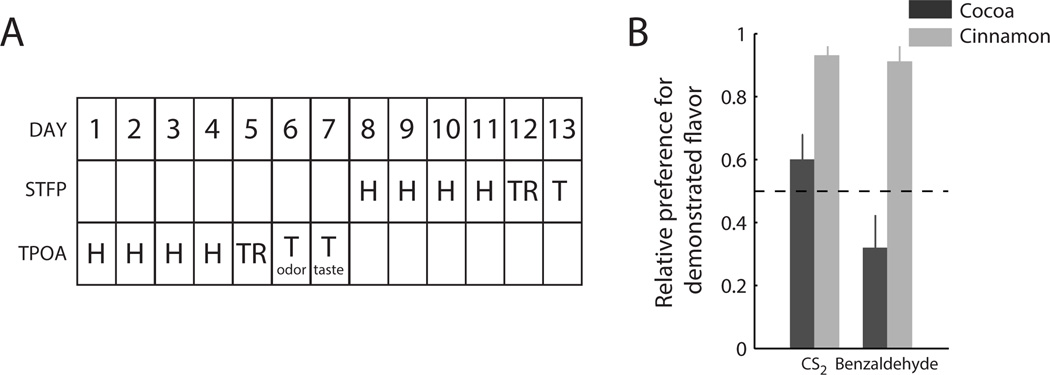
A. Experimental design and time course of Experiments I and II. Animals were first subjected to the TPOA protocol, followed by the STFP paradigm. B. Relative preference for demonstrated flavors of animals that previously learned conditioned aversions to CS2 and benzaldehyde in Experiment I (mean ± SEM). Overall, animals showed significant preferences for the demonstrated flavor. No significant difference in preference between the CS2 and benzaldehyde groups was observed.
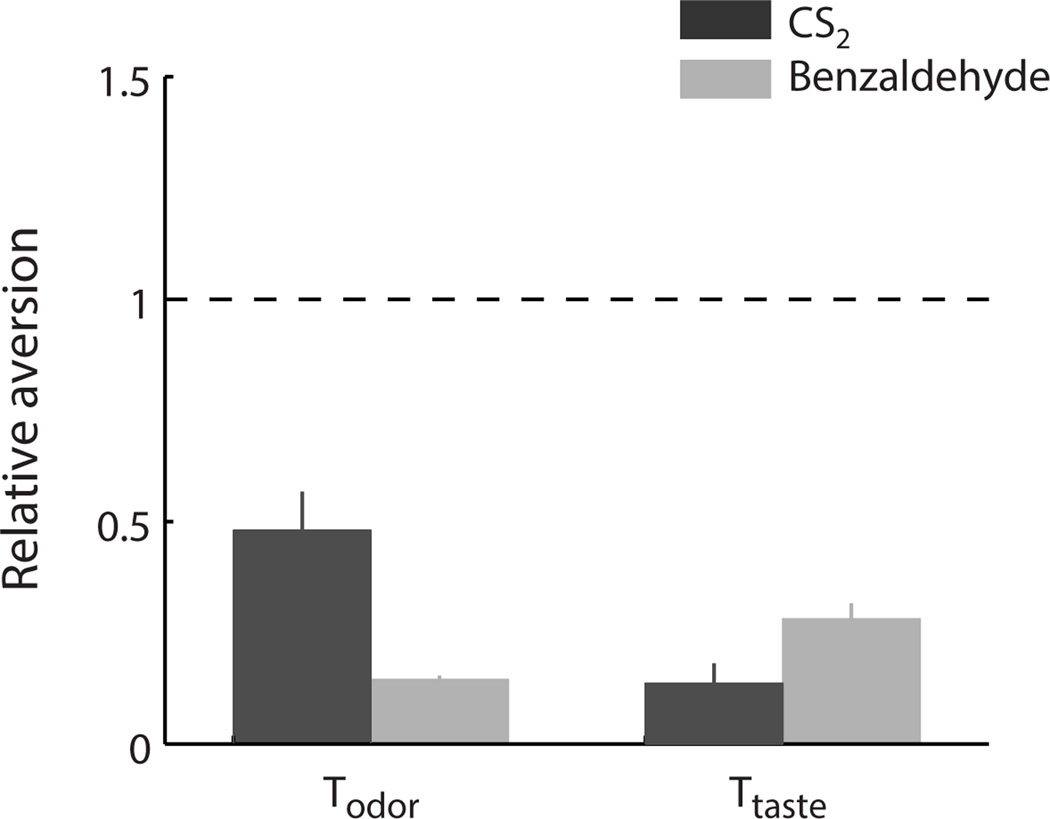
Figure 1 shows relative aversion during testing sessions. Animals exhibited the previously observed (Palmerino et al., 1980; Rusiniak et al., 1979) aversion to benzaldehyde, as reflected in a decreased consumption relative to training (dashed line; t-test: t11=97.8, p<0.001). Animals also exhibited an aversion to CS2 (t16=5.9, p<0.001), although aversions to CS2 were significantly weaker than aversions to benzaldehyde (t27=3.2, p<0.01). Animals also showed the expected aversion to the taste stimulus (saccharin) used in compound with both benzaldehyde (t11=5.9, p<0.001) and CS2 (t16=19.9, p<0.001).
Figure 1.
Consumption during testing sessions relative to training session in the benzaldehyde and CS2 groups (mean ± SEM). Animals’ consumption in both odor and taste testing sessions was significantly decreased compared to the training session in both groups. Aversions to benzaldehyde were significantly stronger compared aversions to CS2.
The findings from Experiment I demonstrate that conditioning can induce an aversion to the semiochemical CS2, and thus that experience can affect how semiochemicals are perceived. The fact that aversions conditioned to CS2 seem relatively weak when compared to aversions to benzaldehyde could be explained by different physical properties, most notably volatility: CS2 is 600 times more volatile than benzaldehyde (vapor pressureCS2=300 versus vapor pressurebenzaldehyde=0.5 [at 20° C]), and thus likely diffuses away from the source and into the rest of the experimental chamber much more quickly; given the relatively strong smell of CS2 at those concentrations, it is reasonable to suspect that the association between the semiochemical and its source was milder than that between benzaldehyde and its source.
Experiment II
Having established that we can make CS2 aversive to the rat, we next asked whether the function of CS2 as a semiochemical was affected by these aversions, by subjecting animals that had undergone the TPOA experiment to a social transmission of food preferences (STFP) paradigm (see Figure 2A). In STFP, observer (subject) animals interact with a demonstrator rat that has recently consumed a novel flavored food immediately preceding the interaction phase (Fortis-Santiago et al., 2010; B. G. Galef & Wigmore, 1983). Since CS2 on the breath of the demonstrator rat has been suggested to mediate observer animals’ preference for the demonstrated flavor via its function as a semiochemical (B. Galef et al., 1988; Munger et al., 2010), the degree to which such preference learning is preserved in rats previously rendered averse to CS2 versus benzaldehyde allows us to test whether this function is preserved following aversion learning.
Figure 2B shows the results of this test—relative preference for the demonstrated flavors displayed by animals that had previously learned an aversion to CS2 or benzaldehyde in the TPOA paradigm. Although the two demonstrated flavors have different intrinsic palatability (cinnamon is preferred over cocoa) overall, animals showed a significant general increase in preference for the demonstrated flavor after training (data from cocoa and cinnamon-demonstrated flavors combined: t26=3.6, p<0.01). This increase was comparable in magnitude to those observed in previous studies using the STFP paradigm (Fortis-Santiago et al., 2010). This learnt preference is furthermore indistinguishable from preferences developed by animals that previously learnt aversions to benzaldehyde: ANOVA on relative preference with factors Odor (CS2 versus benzaldehyde) and Flavor (cinnamon versus cocoa) showed only a significant main effect of Flavor (F(1,26)=40.0, p<0.001), reflecting different intrinsic palatability of cinnamon and cocoa.
Together, the data from Experiments I and II indicate that preference learning in the STPF paradigm is preserved in animals that had previously formed an aversion to CS2.
Experiment III
In experiments I and II, TPOA testing and STFP training were separated by 5–6 days (see Figure 2A). It is thus possible that aversions to CS2 induced by the TPOA paradigm disappeared by the time animals underwent the STFP training session, and that the success of the STFP training relied on that fading of CS2 aversion. For experiment III, we therefore developed a novel protocol whereby a new group of 13 rats performed both paradigms simultaneously. STFP sessions took place in the morning (8–10 AM), and were followed by TPOA sessions in the afternoon (3–5 PM; see Figure 3A). This overlapping design ensured that aversion testing took place within one day of STFP testing, and thus that animals were averse to CS2 during learning of socially transmitted food preferences.
Figure 3.
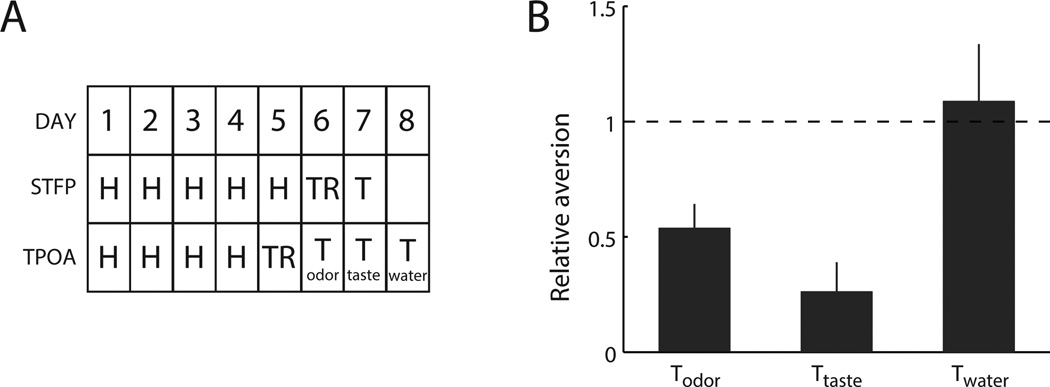
A. Experimental design and time course of Experiment III. Rats were subjected to the TPOA and STFP paradigms simultaneously. B. Consumption (relative to training) during testing sessions for animals in Experiment III (mean ± SEM). Animals’ consumption in both odor and taste testing sessions was significantly decreased compared to training, and not significantly different from aversions observed in Experiment I. Consumption during the control testing session was not different from training.
Figure 3B, which plots amount of fluid consumed during testing sessions relative to consumption during the training session for the group of animals that underwent the TPOA and STFP paradigm simultaneously, shows that the “simultaneous” training regime did not interfere with the learning of aversions. In fact, aversions to CS2 observed for this group were indistinguishable from those observed in Experiment I (t28=0.4, p=0.67), as were aversions to saccharin (t27=1.1, p=0.30).
We also performed an additional control, testing whether the observed decrease in consumption during testing sessions was the result of animals forming an aversion to the testing environment itself, rather than to CS2 and saccharin. First, animals were given access to the recovery bottle (containing 10 ml of water, see Methods) in their test cages, one hour after each TPOA session. All animals readily drank the full amount of water during these sessions, even after the training session. Second, we tested consumption of water in the absence of CS2 during a third testing session (see Figure 3B): no significant aversion was observed in this session (t11=0.4, p=0.73). These findings confirm that animals did not form an aversion to the testing environment, and that aversions observed during odor and taste testing sessions are indeed stimulus specific.
Despite having an aversion to CS2, preference learning in the STFP paradigm, taking place on the same day aversions were assessed, was indistinguishable from preference learning observed after aversive conditioning to CS2 in Experiment II. ANOVA with factors Design (separate experiments versus simultaneous experiments) and Flavor (cinnamon versus cocoa) showed only a significant main effect of Flavor (F(1,29)=17.1, p<0.001), reflecting again different intrinsic palatability of cinnamon and cocoa and demonstrating that rats develop strong food preferences based on the semiochemical properties of CS2 despite having a learned aversion to CS2 itself..
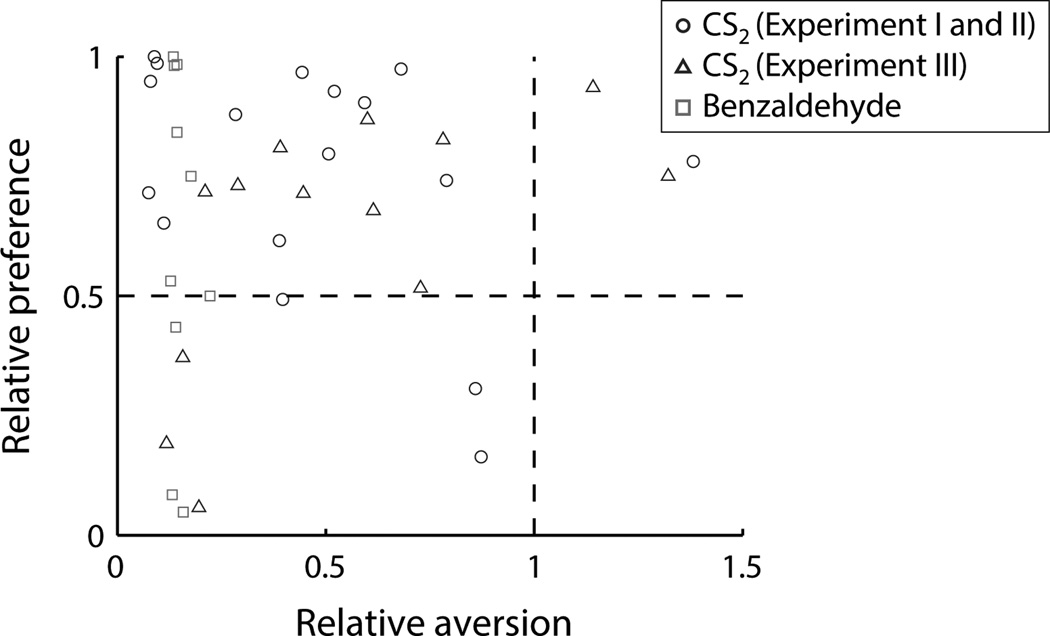
In fact, the strength of food preference learned in the STFP task was unaffected by the aversion to CS2 learned in the TPOA task. Odor aversions and food preferences for all animals in the CS2 and benzaldehyde groups are shown in Figure 4. Most data points fall below 1 on the aversion axis, reflecting aversions to the odor stimuli, and above 0.5 on the preference axis, indicating a preference for the demonstrated flavor. There was no correlation between the strength of aversions and preferences (CS2: r=0.05, p=0.79; benzaldehyde: r=−0.12, p=0.74).
Figure 4.
Relative preferences observed in the STFP paradigm plotted against relative aversions to CS2 or benzaldehyde observed in the TPOA paradigm for all animals. No significant correlations between the two measures were observed.
The data from Experiment III confirm that STFP learning is unimpaired, even in rats that are averse to CS2, suggesting that the semiochemical CS2 processing stream functions independently of the canonical stream whereby rats learn about odors.
Discussion
Here, we have provided evidence that the semiochemical CS2 is processed via multiple parallel channels. First, we showed that perception of CS2 is susceptible to aversive conditioning in the context of a taste-potentiated odor aversion paradigm, despite its pheromonal properties; we then went on to show that this attempt to devalue the inherent value of CS2 had no effect on its ability to function as an unconditioned stimulus in mediating socially transmitted food preferences.
Our results indicate that at least part of the neural circuitry activated by CS2 is subject to experience-dependent plasticity, while leaving semiochemical processing of CS2 intact. The importance of these results can perhaps be better appreciated via comparison with more commonly-appreciated “devaluation” studies, which are highly similar in form but different in result: when sucrose is rendered aversive via conditioned taste aversion, it typically loses its ability to drive or sustain learning (even learning with very different contextual parameters), and previous conditioning for which sucrose had been the US becomes attenuated (Yin & Knowlton, 2002); creation of an aversion to CS2, meanwhile, utterly fails to “devalue” that CS2 in STFP.
This pattern of results demonstrates that processing of semiochemicals—or at least CS2—is mediated by neural circuitry that is distinct from those circuits responsible for the processing of more canonical stimuli. An obvious neural substrate for this distinction can be found at the periphery—before neural processing even begins. In rodents, the semiochemical properties of CS2 are processed by a highly sensitive and selective subsystem within the main olfactory system that includes olfactory sensory neurons (OSNs) expressing guanylyl cyclase D (GC-D) receptors. Genetic disruption of the GC-D transduction cascade (Gucy−/− mice) results in a failure to acquire socially transmitted food preferences. However, high concentrations of CS2 evoke residual responses in the olfactory epithelium of Gucy−/− mice, suggesting that CS2 can activate canonical OSNs in addition to GC-D OSNs (Munger et al., 2010).
The present results are satisfactorily explained in terms of these two neural processing streams. Perhaps the processing of CS2 as a canonical odorant is subject to experience-dependent plasticity, whereas semiochemical processing of CS2, which originates in transduction through the GC-D pathway and is thought to be primarily responsible for transmitting food preferences, is not.
Related to this possibility is the fact that the two paradigms make use of very different concentrations of CS2—the concentration in rat breath (<1 ppm) (B. Galef et al., 1988) is only a small fraction of that available during TPOA. GC-D OSNs are sensitive enough to respond to the lower concentration, but canonical OSNs require much higher concentrations of CS2 (Munger et al., 2010). It is thus possible that during the social interaction phase of the STFP paradigm, only GC-D OSNs are activated, whereas during TPOA concentrations of CS2 were high enough (between 10 and 100 ppm) to stimulate canonical OSNs, allowing learning-related plasticity. If the two pathways are indeed distinct from transduction, this fact would help to ensure stability of STFP in the face of TPOA-related plasticity.
Of course, there is any number of other differences between the two behavioral paradigms—just two examples are the use of fluids versus solid foods during testing sessions, and the use of CS2 as a conditioned and unconditioned stimulus—that could also play into our results to some degree. It is well known that even subtle contextual changes can have a significant impact on the nature of neural chemosensory processing (see, for instance Beshel, Kopell, & Kay, 2007; Kay & Beshel, 2010), even changing the networks involved in processing (Fontanini & Katz, 2006; Veldhuizen, Douglas, Aschenbrenner, Gitelman, & Small, 2011); perhaps one or several of these factors underlie the fact that STFP processing appears to be independent of the pathways changed by TPOA. Either way, the results imply a separation of pathways. There are at least two alternative explanations that we cannot conclusively rule out. First, there are multiple sulfur compounds other than CS2 (including carbonyl sulfide B. Galef et al., 1988) available in rat breath, and we cannot exclude the possibility that one of these other semiochemicals might take over the signaling function of CS2 after aversive conditioning to CS2. We consider this possibility remote, however, for the simple reason that we are unaware of a precedent for such a phenomena. Second, there is the fact that, in STFP, CS2 is experienced in mixture with a food odor, whereas in TPOA, CS2 is experienced in isolation; it is possible that the very act of mixing CS2 with another odor eliminates its conditioned aversiveness. If this accounts for dual perception of CS2, one would expect TPOA to CS2 to be absent when presented in mixture during testing. This possibility is also unlikely, however, in the light of work on conditioned taste version, where aversions to tastes tested in mixture with other tastes are reduced, but not eliminated (Domjan, 1975). In the absence of a second processing pathway, the STFP for CS2-averse rats should still have been smaller than normal, which it was not.
The adaptive value of independent olfactory pathways lies in that it ensures co-existence of both modifiable (canonical) olfactory processing mechanisms that are subject to experience-dependent plasticity, and stable semiochemical systems, without stable semiochemical function being affected by experience. Future work will shed light on the influence of contextual factors and neural processing systems on dual perception of CS2 by testing whether aversion and preference behaviors can be independently disrupted by selective manipulation of context and/or neural pathways.
Acknowledgments
Many thanks to Christine McInnis, Mike Rakoski and Madelen Diaz for help with data collection. This work was supported by NIH DC 7703 (to DBK).
References
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27(31):8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne TH, Johnston AN, Wilkinson LS, Kendrick KM. Effects of anesthetic agents on socially transmitted olfactory memories in mice. Neurobiol Learn Mem. 2010;93(2):268–274. doi: 10.1016/j.nlm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Domjan M. Poison-Induced Neophobia in Rats - Role of Stimulus Generalization of Conditioned Taste Aversions. Animal Learning & Behavior. 1975;3(3):205–211. doi: Doi 10.3758/Bf03213432. [Google Scholar]
- Fontanini A, Katz D. State-dependent modulation of time-varying gustatory responses. J Neurophysiol. 2006;96(6):3183–3193. doi: 10.1152/jn.00804.2006. doi: DOI 10.1152/jn.00804.2006. [DOI] [PubMed] [Google Scholar]
- Fortis-Santiago Y, Rodwin BA, Neseliler S, Piette CE, Katz DB. State dependence of olfactory perception as a function of taste cortical inactivation. Nat Neurosci. 2010;13(2):158–159. doi: 10.1038/nn.2463. 10.1038/nn.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Jr, Wigmore SW, Kennett DJ. A failure to find socially mediated taste aversion learning in Norway rats (R. norvegicus) J Comp Psychol. 1983;97(4):358–363. [PubMed] [Google Scholar]
- Galef B, Mason J, Preti G, Bean N. Carbon-disulfide - a semiochemical mediating socially-induced diet choice in rats. Physiol Behav. 1988;42(2):119–124. doi: 10.1016/0031-9384(88)90285-5. [DOI] [PubMed] [Google Scholar]
- Galef BG, Wigmore SW. Transfer of information concerning distant foods - a laboratory investigation of the information-center hypothesis. Anim Behav. 1983 Aug;31:748–758. [Google Scholar]
- Hishimura Y. Re-examination of the poisoned-partner effect with the two-bottle testing method. Behav Processes. 2000;50(2–3):95–99. doi: 10.1016/s0376-6357(00)00093-0. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317(5840):953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Iraola JA, Alonso G. The influence of flavored solution concentration on the poisoned-partner effect. Neurobiol Learn Mem. 1995;63(2):149–154. doi: 10.1006/nlme.1995.1015. [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophysiol. 2010;104(2):829–839. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104(36):14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405(6788):792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442(7103):645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434(7032):470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24(14):3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299(5610):1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 2009;101(5):2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P, Kelliher KR. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol. 2010;20(16):1438–1444. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiology and Behavior. 1973;10(1):73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- Palmerino CC, Rusiniak KW, Garcia J. Flavor-illness aversions: the peculiar roles of odor and taste in memory for poison. Science. 1980;208(4445):753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- Rusiniak KW, Hankins WG, Garcia J, Brett LP. Flavor-illness aversions - potentiation of odor by taste in rats. Behav Neural Biol. 1979;25(1):1–17. doi: 10.1016/s0163-1047(79)90688-5. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Westbrook F, Darling FMC. What the rat's nose tells the rat's mouth: Long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Animal Learning and Behavior. 1997;25(3):357–369. doi: Doi 10.3758/Bf03199093. [Google Scholar]
- Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. J Neurosci. 2011;31(41):14735–14744. doi: 10.1523/JNEUROSCI.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489(4):491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Reinforcer devaluation abolishes conditioned cue preference: evidence for stimulus-stimulus associations. Behav Neurosci. 2002;116(1):174–177. doi: 10.1037//0735-7044.116.1.174. [DOI] [PubMed] [Google Scholar]