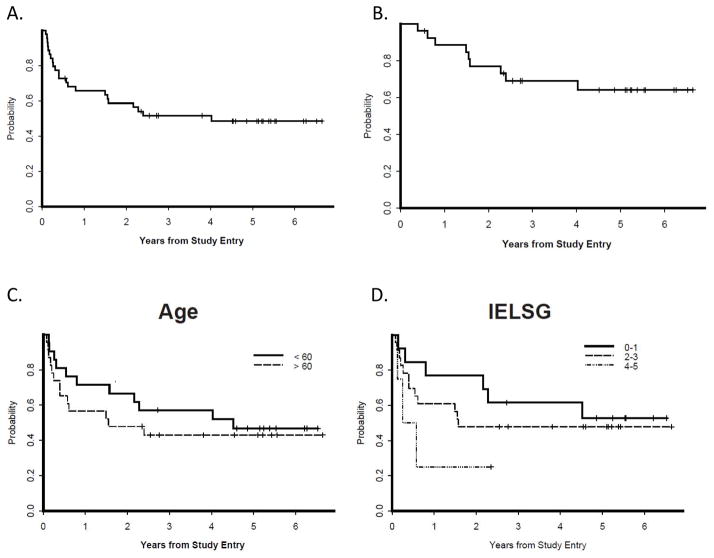
Figure 5. Outcomes with intensive chemotherapy and immunotherapy in newly-diagnosed primary central nervous system lymphoma, without whole brain radiotherapy: CALGB (Alliance) 50202).
Outcome for all CALGB 50202 patients; y-axis refers to cumulative probability of event. 4A. Progression-free survival (PFS) for all patients. The 2-year PFS was 59%. 4B. PFS for those patients who attained a complete response with MT-R (high-dose methotrexate, temozolomide, rituximab) induction and received EA (etoposide cytararbine) consolidation (n=27). 4C. PFS was similar for patients aged> 60 years (n=23) and for younger patients (n=21; p=0.48). 4D. There was a trend between shorter PFS and highest International Extranodal Lymphoma Study Group (IELSG) risk score of 4–5 (p=0.16).