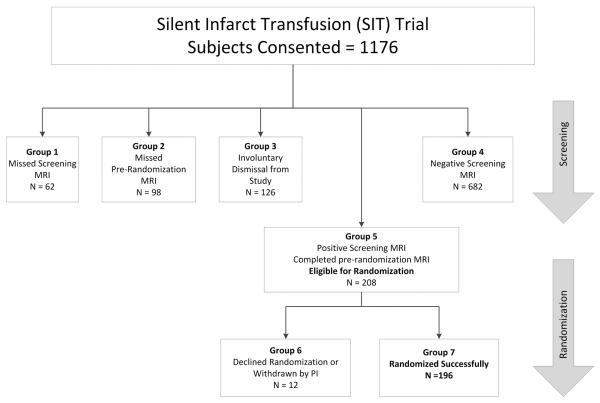
Figure 1. Study Participation and Withdrawal in the SIT Trial.
Participants in the SIT Trial underwent both an initial screening and pre-randomization MRI. Those with SCI on screening MRI but no progression of disease on pre-randomization MRI or transcranial Doppler were subsequently randomized to transfusions or observation. Groups were defined as: 1) consented participants who missed initial screening MRI, 2) consented participants who had a positive screening MRI but missed pre-randomization MRI, 3) consented participants who were involuntarily dismissed from the study due to abnormal transcranial Doppler ultrasound or progression of disease on pre-randomization MRI and those with MRI failures, 4) consented participants who screened negative on initial MRI, 5) all participants who successfully completed screening MRI, pre-randomization MRI and were eligible for randomization; 6) participants who were eligible for randomization but either declined randomization or were dismissed from the study due to non-adherence, lost to follow-up, etc., and 7) consented participants who were successfully randomized.