Abstract
The Gulf-Coast tick, Amblyomma maculatum, possesses an elaborate set of selenoprotein, which prevent the deleterious effects from oxidative stress that occur during feeding. In the current work, we examined the role of Selenoprotein K (SelK) and Selenoprotein M (SelM) in feeding A. maculatum by bioinformatics, transcriptional gene expression, RNA interference and antioxidant assays. The transcriptional expression of SelK does not vary significantly in salivary glands or midguts throughout the blood meal. However, there is a 58-fold increase in transcript levels of SelM in tick midguts. Ticks injected with selK-dsRNA or selM-dsRNA did not reveal any observable differences in egg viability but oviposition was reduced. Surprisingly, salivary antioxidant activity was higher in selenoprotein knockouts compared to controls, which is likely due to compensatory transcriptional expression of genes involved in combating reactive oxygen species. In fact, RT-qPCR data suggest the transcriptional expression of catalase increased in ticks injected with selM-dsRNA. Additionally, the transcriptional expression of selN decreased ~90% in both SelK/SelM knockdowns.
Keywords: Selenoprotein K, Selenoprotein M, tick antioxidants, oxidative stress
Introduction
Ticks are obligate ectoparasites and imbibe a huge volume of blood, equivalent to approximately 100 times their unfed weight which requires the tick to concentrate the blood meal and subsequently regurgitate excess fluid (Bezuidenhout 1987; Brown 1988). In fact, ticks transmit the greatest variety of pathogens, second only to mosquitoes in terms of impact on human health. The Gulf-Coast Tick, Amblyomma maculatum, harbors Rickettsia parkeri which can cause a mild, febrile illness similar to Rocky Mountain spotted fever (Paddock et al. 2004). Pathogen transmission is enhanced by tick saliva, a remarkably complex mélange including anti-inflammatory proteins, anticoagulants, antihistamines, lectins, and cement proteins which are critical for prolonged attachment to the host (Munderloh et al. 2005). Ticks encode a number of antioxidants, to cope with the host defense system, for the digestion of heme, or to counteract reactive oxygen species.
Reactive oxygen species (ROS) are produced by many cellular processes and enzymes such as mitochondrial oxidative phosphorylation, NADH/NADPH oxidase, P-450 monooxygenase, lipoxygenase, cyclooxygenase, xanthine oxidase and are primarily mitigated by selenoproteins (Reeves and Hoffmann 2009). Glutathione peroxidases (GPx) detoxify lipid peroxides using reduced glutathione as an electron donor (Das et al. 2001) while thioredoxin reductase (TrxR) is responsible for regenerating the reduced thioredoxin using NADPH as an electron donor (Sandalova et al. 2001). Thioredoxin is used by several enzymes in dithiol-disulfide exchange reactions. Selenoproteins are present in bacteria, archaea, and eukaryotes, and exhibit a diverse pattern of localization and expression (Kryukov et al. 2003). The number of selenoproteins varies widely, from 10–57 in algae, 30–37 in fish, and 23–25 in mammals, but are not universal, particularly so in arthropods (Lobanov et al. 2009; Mariotti et al. 2012). Some insect species possess cysteine-containing homologs or may lack selenoproteins altogether, such as thioredoxin reductase and glutathione peroxidases, which are essential in mammalian systems (Shchedrina et al. 2011b). The reduction in selenoproteins can in part be attributed to the simplicity in the conversion of a Sec to a Cys codon, which requires only a single point mutation (Mariotti et al. 2012). Recent studies have shown that at least five insect species do not contain the cellular machinery to incorporate selenocysteine into selenoproteins: Tribolium castaneum, Bombyx mori, Drosophila willistoni, Apis mellifera and Nasonia vitripennis (Chapple and Guigo 2008; Lobanov et al. 2008). Moreover, the role of selenoproteins in Drosophila does not appear to be critical for lifespan or oxidative stress defense (Hirosawa-Takamori et al. 2004).
Tick selenoproteins have been barely investigated, but there is evidence that suggests they may play critical roles in the pathogen cycle. Glutathione peroxidase (Salp25D) in Ixodes scapularis saliva plays its well-characterized role in the peroxide detoxification but also was found to be important in the acquisition of Borrelia burgdorferi spirochetes from murine hosts (Narasimhan et al. 2007). One study has shown that the expression of SelM is upregulated in Dermocenter variabilis infected with Anaplasma marginale, and infection levels in tick guts were reduced after the depletion of selM transcript (Kocan et al. 2009). In the salivary glands of the hard tick Hyalomma asiaticum asiaticum, Hyalomin–A and –B were found to suppress host inflammatory responses by modulating cytokine secretion and detoxifying reactive oxygen species (Wu et al. 2010).
Our laboratory is investigating the impact of antioxidants within the framework of tick feeding, oviposition, and R. parkeri acquisition, maintenance, trafficking, and transmission (Adamson et al., 2013). SelM and SelK represent two interesting candidates for further study. As previously mentioned, SelM has been previously shown to respond to pathogen infection in D. variabilis (Kocan et al. 2009). SelK is an ER membrane protein important for Ca2+ influx during the activation of immune cells and has also recently been shown to be a target for m-calpain, a calcium-activated cysteine protease which regulates inflammation and immune responses (Huang et al. 2011; Verma et al. 2011). Determining the cellular role within pathogen-free ticks will contribute to their impact of antioxidants and lay the groundwork for future studies in R. parkeri-infected ticks.
In this preliminary study, we examined the cellular function of SelK and SelM in A. maculatum using RNA interference. It is apparent that selenoproteins K or M are not essential to feeding, vitellogenesis or fecundity, but egg masses were smaller. The transcriptional expression of catalase increased in the SelM knockdown, while the transcriptional expression of SelN decreased in both SelK and SelM knockdowns. Antioxidant assays using saliva collected from gene knockdown ticks demonstrate higher levels of antioxidant activity than in controls. Taken together, these data suggest that a robust compensatory mechanism exists in ticks to overcome selenoprotein deficiency.
Results and Discussion
Bioinformatic analysis
SelK belongs to the DUF2763 superfamily with no known function. SelK homologs of AmSelK were initially identified by BLASTP analysis of the non-redundant protein database yielding 28 initial hits with an E-value <1E−6 (Altschul et al. 1990). SelM belongs to the Sep15/SelM superfamily which contains a thioredoxin-like domain and a surface accessible active site redox motif. This suggests that SelM functions as a thiol-disulfide isomerase involved in disulfide bond formation in the endoplasmic reticulum (Ferguson et al. 2006). SelM homologs of AmSelM identified by BLASTP analysis of the non-redundant protein database yielded 35 initial hits with an E-value <1E−6.
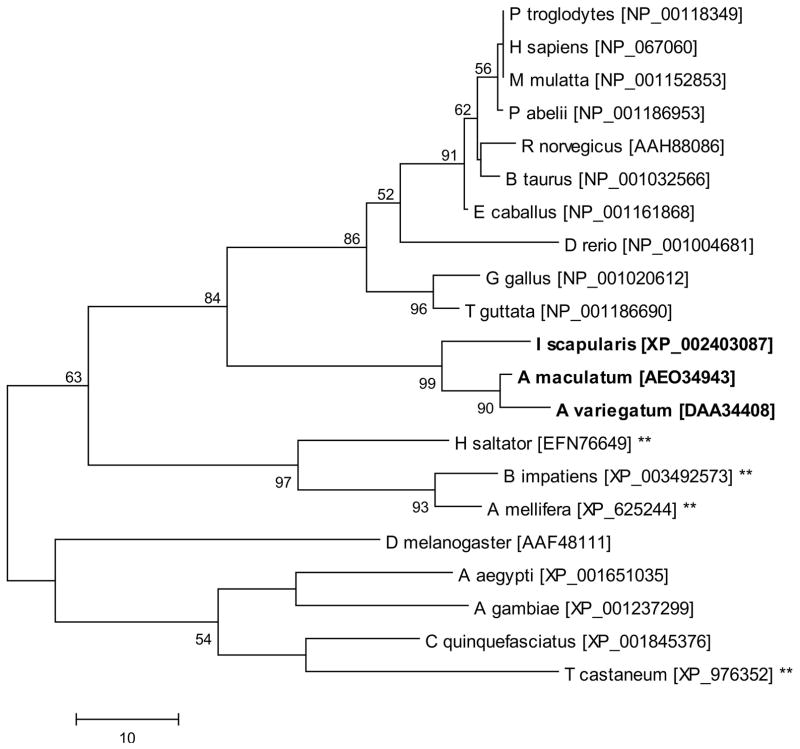
The homology of SelK and SelM, and other representative vertebrate and invertebrate species deduced amino acid sequences was explored by multiple sequence alignment and phylogeny. Like other representative sequences, tick SelK sequences are not predicted to be secreted and are predicted to contain a membrane-spanning region by the DAS transmembrane prediction server (Cserzo et al. 2004; Cserzo et al. 1997). This data is in agreement with previous reports suggesting that SelK is a transmembrane protein localized to the ER (Du et al. 2010). Similar to other sequences, tick SelK possesses a selenocysteine residue encoded in the C-terminus (Fig. 1). Tick SelK sequences share 77% amino acid identity with each other but share slightly higher amino acid identity to H. sapiens (38–40%) than to D. melanogaster SelK sequences (30–32%) (Fig. 1). Arthropod and higher-order vertebrate SelK sequences were well separated in the dendrogram, with the tick SelK sequences more similar to higher-order vertebrate sequences than to other arthropods (Fig. 3).
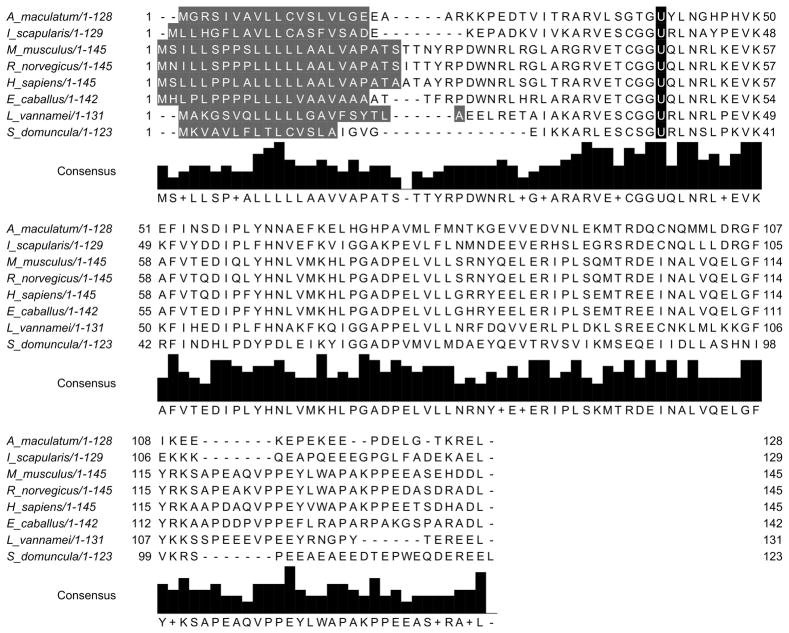
Figure 1.
The multiple sequence alignment of Selenoprotein K. The selenocysteine residue (U) is highlighted in black.
Figure 3.
The evolutionary history of Selenoprotein K was inferred using the Maximum Parsimony method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Tick sequences are indicated in bold and sequences denoted with asterisks are derived from species which lack the capacity to synthesize selenoproteins and represent cysteine-containing homologs. Scale bar represents amino acid substitutions per position.
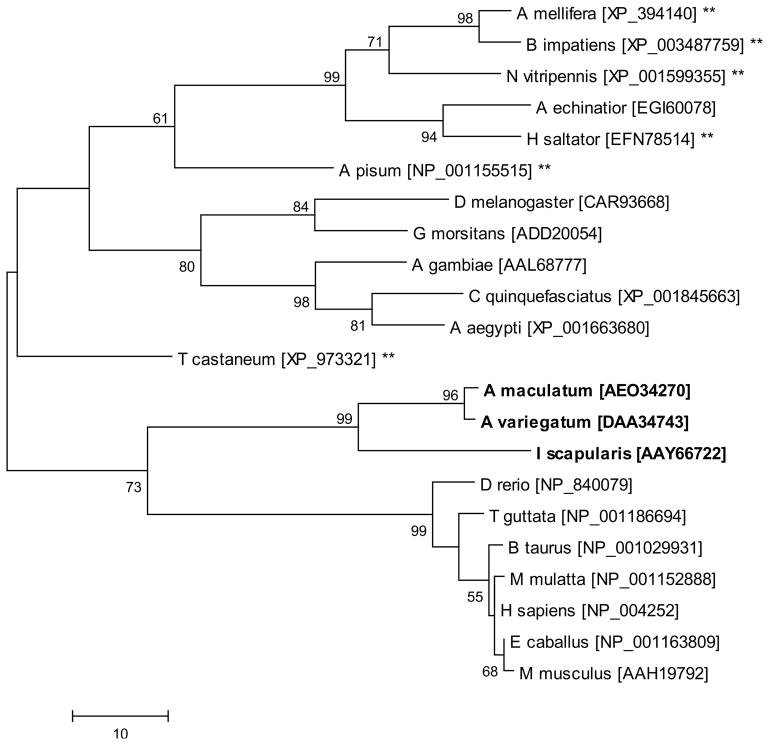
Tick SelM sequences are predicted to have a signal peptide, targeting the protein for secretion, which is similar to all other orthologs studied. Tick SelM sequences share only 47% amino acid identity to each other and 28–35% identity with SelM from H. sapiens (Fig. 2), and in all examined cases, the selM gene encodes a selenocysteine residue in the N-terminal region of the mature protein. SelM sequences were more closely related to higher-order vertebrate SelM sequences than to other arthropods (Fig. 4).
Figure 2.
The multiple sequence alignment of Selenoprotein M. The selenocysteine residue (U) is highlighted in black. Selenoprotein M is predicted to have a signal peptide (highlighted dark grey), targeting the protein for secretion.
Figure 4.
The evolutionary history of Selenoprotein M was inferred using the Maximum Parsimony method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Tick sequences are indicated in bold and sequences denoted with asterisks are derived from species which lack the capacity to synthesize selenoproteins and represent cysteine-containing homologs. Scale bar represents amino acid substitutions per position.
In both cases, the tick SelK and SelM sequences had considerable evolutionary divergence from cysteine-containing homologs present in many of the insects (Figs. 3 and 4). These data suggest strongly suggest that conservation of the selenocysteine residue is important in at least these two tick species. This may be related to the higher chemical reactivity of selenocysteine residues compared to their cysteine homologs. Selenocysteine and cysteine are identical amino acids, except for a single atom (Se vs. S), but, selenium has a much lower pKa (5.2 vs 8.3) and higher reactivity, which explains why selenoproteins possess ~100-fold higher catalytic efficiency than their cysteine-containing homologs (Reeves and Hoffmann 2009).
Transcriptional expression throughout the blood meal
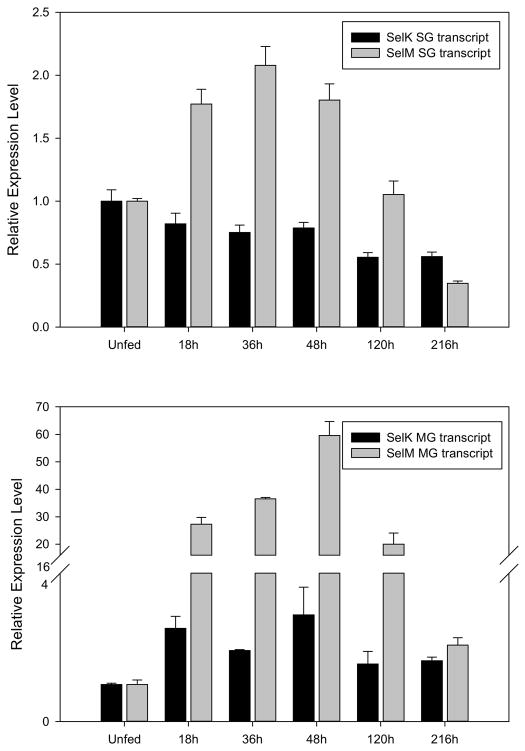
Quantitative RT-PCR was employed to investigate the transcriptional expression of SelK and SelM in tick midguts and salivary glands throughout the blood meal and these values are compared to levels observed in unfed ticks. In salivary glands, the transcriptional expression of SelK slowly decreased throughout the blood meal, with highest levels observed in unfed ticks, dropping to 55% of the levels observed in unfed ticks at 216hr post-infestation (Fig. 5). The transcriptional expression of SelK is considerably more dynamic in midguts, where it increases three-fold compared to unfed ticks, peaking at 48hr after the onset of blood feeding, but remaining high throughout the blood meal (Fig. 5). Previous studies have shown that the human SelK gene contains a functional ER stress response element and its expression was up-regulated by conditions that induce the accumulation of misfolded proteins (Du et al. 2010). Moreover, SelK co-precipitated p97 ATPases, Derlins, and SelS, which are part of the ER-associated degradation (Shchedrina et al. 2011a). This suggests that if this ER stress response element is present in the tick SelK gene then it may operate to prevent the accumulation of misfolded proteins resulting from ER-derived oxidative stress in feeding tick salivary glands (Malhotra and Kaufman 2007).
Figure 5.
The transcriptional gene expression of selK and selM in the salivary glands (top) and midguts (bottom) of female A. maculatum ticks throughout the blood meal. The gene expression of SelK and SelM was normalized to the unfed developmental stage using β-Actin as a reference gene.
The transcriptional expression of SelM in salivary glands increased to 2-fold higher than unfed ticks by 36hr post-infestation, and then sharply dropped off to 35% of the levels observed in unfed ticks by 216hr post-infestation (Fig. 5). Surprisingly, very little SelM transcriptional activity was observed in unfed tick midguts, and once blood feeding commenced, the transcriptional expression rose 59-fold and but fell to similar levels observed in unfed tick midguts (Fig. 5). The function of SelM is not yet clear, but a related selenoprotein protein, Sep15, shares 31% sequence identity. Both SelM and Sep15 have an α-/β-fold with a central β-sheet surrounded by α-helices, which is typical of thioredoxin-like proteins (Ferguson et al. 2006). They also both undergo conformational changes after thiol-disulfide exchange and have sequence homology to protein disulfide isomerases, which suggest that these two proteins function as thiol-disulfide oxidoreductases (Ferguson et al. 2006). Sep15 associates with UDP glucose:glycoprotein glucosyltransferase (UGTR) in the ER, which is involved in the quality control of protein folding, and it is possible that SelM functions in a similar manner, though data is still lacking to support this (Korotkov et al. 2001; Labunskyy et al. 2005). The rapid rise in the transcriptional expression of SelM within 48-hours post-infestation, suggests that events occurring while the tick is feeding places a large burden on protein synthesis and protein folding machinery.
RNA interference
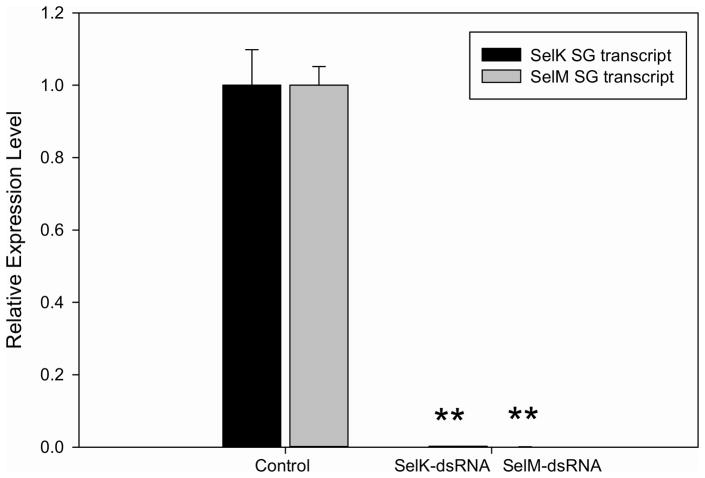
Injecting selK or selM into the body of A. maculatum adult female ticks were reduced by 99% of the transcript in salivary glands sampled at seven days post-infestation, compared to gfp-dsRNA control (Fig. 6). Oviposition was reduced and eggs were somewhat brown in color (Fig. 7), which is consistent with previous studies demonstrating that oxidative stress reduced egg-laying in Rhipicephalus microplus (Citelli et al. 2007). No differences in hatching rate were noted between control and experimental groups. Similarly, the depletion of catalase and a corresponding increase in reactive oxygen species was shown to reduce the fecundity of phlebotomine sand flies, Lutzomyia longipalpis (Diaz-Albiter et al. 2011). Taken together, these data suggest that tick antioxidants could be important prior to embryogenesis, possibly by ameliorating the harmful effects of heme digestion, which would otherwise reach cytotoxic levels in the feeding tick (Citelli et al. 2007; Graca-Souza et al. 2006).
Figure 6.
Quantitative PCR showing transcriptional expression of SelK and SelM in A. maculatum salivary glands of control and SelM- or SelK-dsRNA and GFP-dsRNA (control) injected ticks. Samples were obtained seven days post-infestation. Expression was normalized against the β-Actin. Asterisks indicate a significant difference compared to control (**p≤0.001).
Figure 7.
Photographs of the oviposition of ticks injected with selenoprotein-dsRNA or control ticks.
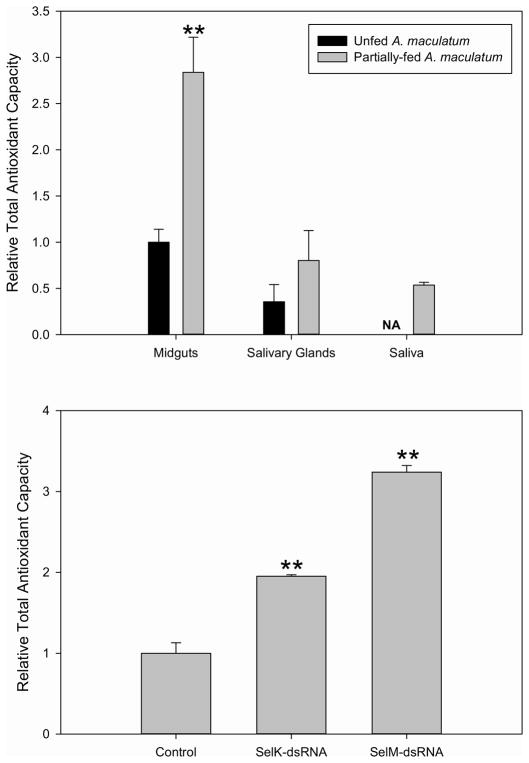
Tick saliva was collected from selenoprotein knockdowns and assayed for total antioxidant capacity to determine the potential role in SelK and SelM in maintaining low levels of cellular reactive oxygen species. Saliva collected from SelM knockdown ticks had 324% of the total antioxidant capacity when normalized to mock-injected ticks (Fig. 8). Saliva collected from SelK-knockdown ticks had 195% of the total antioxidant capacity when normalized to mock-injected ticks (Fig. 8), which is somewhat surprising since SelK has no predicted secretory peptide and was not expected to substantially affect antioxidant levels in tick saliva (Du et al. 2010; Shchedrina et al. 2011a). It has previously been reported that there is some degree of functional redundancy for selenoproteins, and therefore, abolishing the transcript of one selenoprotein may be overcome by expression of other antioxidants (Makarova et al. 1999; Rederstorff et al. 2011; Verma et al. 2011; Wirth et al. 2010).
Figure 8.
The relative total antioxidant capacity was determined in extracts of tick tissues (top) and in pooled saliva collected from ticks injected with selenoprotein-dsRNA compared to control (GFP-dsRNA) (bottom) collected from tissue seven days post-infestation. Asterisks indicate a significant difference compared to control (**p≤0.001). The total antioxidant capacity within saliva of unfed ticks was not determined due to difficulties in collecting sufficient saliva to perform experimentation.
Selenoprotein transcriptional expression in knockdowns
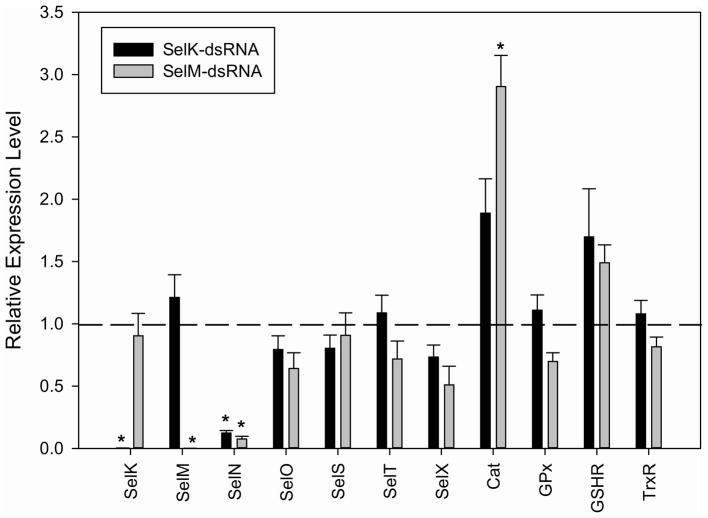
Due to the possible compensatory transcriptional activation of other selenoproteins, we determined the transcriptional expression of other selenoproteins/antioxidants in the knockdowns. The transcriptional expression of selN decreased 82% and 89% in SelK-dsRNA and SelM-dsRNA knockouts, respectively (Fig. 9; p≤0.05). The transcriptional expression of SelO, SelS, SelT, SelX, GPx (Salp25d), GSHR and TrxR did not significantly change in the SelM or SelK knockouts. The transcriptional expression of catalase increased almost three-fold in the SelM knockdown, suggesting the catalase may exhibit some functional redundancy with SelM. The transcriptional expression of catalase increased almost two-fold in the SelK knockdown, but was slightly (5%) below the defined target of statistical significance, which requires a two-fold change in expression. It is noteworthy that the transcriptional expression of catalase would be affected in the SelM/SelK knockdowns, since catalase is a peroxisomal enzyme, whereas SelK and SelM reside in the endoplasmic reticulum (along with SelI, SelS, Sep15, SelN, GPx, TrxR) (Arbogast and Ferreiro 2010), in which we do not observe any transcriptional up regulation.
Figure 9.
The compensatory effect on selenoprotein transcriptional expression in A. maculatum salivary glands injected with SelK- or SelM-dsRNA. Asterisks indicate a statistically significant difference compared to control (GFP-dsRNA injected) (*p<0.05).
The presence of off-target effects is a central concern in the application of RNAi. This phenomenon originates from changes in genes expression from 1) genes that are affected by cellular machinery to fight viral infection and 2) genes that are unintended targets as a result of the use of long double stranded RNA (Lew-Tabor et al. 2011). This is particularly true, when the complete genomic information is lacking, such as for A. maculatum, which would otherwise facilitate the design of dsRNA fragment which do not impact the expression of other genes. In RNAi, the use of an irrelevant gene (such as GFP) can be used to control for any changes in gene expression that might result from induction of tick antiviral genes. Second, using shorter dsRNA (100–200bp in length) has been shown to limit the influence of off-target effects (Lew-Tabor et al. 2011). In this assay, we used RT-qPCR amplicons which are the ideal length for RNAi applications.
Taken collaboratively, these data indicate an elegant relationship between ER-residing selenoproteins. The functional activity of both SelK and SelM has been proposed to prevent the accumulation of misfolded proteins in the ER. The precise function of SelK has not been determined but it associates with proteins involved in the elimination of misfolded proteins from the ER (Du et al. 2010; Shchedrina et al. 2011a). Selenoprotein M very likely functions as a thiol-disulfide oxidoreductase, due to the presence of a thioredoxin-fold and conformational changes after thiol-disulfide exchange, as well as sequence homology to protein disulfide isomerases (Ferguson et al. 2006; Reeves and Hoffmann 2009). Although direct evidence in vivo is currently not available, SelM has been suggested to play a role in protein-folding in the ER (Labunskyy et al. 2005). There are several reports showing the transcriptional induction of selK or selM under conditions which cause ER stress (Du et al. 2010; Hwang et al. 2008; Labunskyy et al. 2005; Reeves and Hoffmann 2009; Shchedrina et al. 2011a).
The downregulation of SelN in both SelK- and SelM-dsRNA injected ticks is surprising. There are no shared sequence regions between SelN and SelK/SelM, which suggests that the downregulation of SelN is a genuine biological response perhaps even a feedback system, and not simply an off-target effect of SelM/SelK knockdown. It has previously been suggested that SelN may limit ER damage against ROS by regulating protein maturation, folding, trafficking, or stability, as with SelK and SelM, but evidence for this function has yet to be provided (Castets et al. 2012). In muscle tissue, SelN is physically associated with ryanodine receptor, a calcium channel involved in muscle excitation/contraction (Jurynec et al. 2008; Rederstorff et al. 2011). Most interestingly, the sensitivity of the ryanodine receptor to oxidative stress was drastically reduced in SelN-deficient zebrafish mutants and patient biopsies (Arbogast and Ferreiro 2010; Castets et al. 2012; Rederstorff et al. 2011). Perhaps the decrease in SelM and SelK resulted in a redox imbalance in the ER, and therefore transcriptional downregulation of selN (Fig. 9) may prevent Ca2+ overload, leading to further oxidative stress through the activation of calcium-dependent oxidant sources (Klee and Means 2002). This could, in part, explain the relatively high levels of salivary antioxidant capacity in the SelK/SelM knockdowns (Fig. 8).
Taken together, these results suggest that antioxidant defense functions of SelK and SelM known in other species may be extended to include ticks. The elevated levels of total antioxidant activity in saliva of knockdown ticks suggest that a strong compensatory mechanism for maintaining antioxidant activity exists within tick salivary glands. The changes in egg masses of knockdown ticks compared to controls suggest that oviposition or vitellogenesis were impacted by the deficiency in these two selenoproteins, despite the fact that saliva of knockdown ticks had higher antioxidant activity. Future research should focus on elucidating a definitive role of these selenoproteins in many tick species. Further research should evaluate mechanisms by which these genes contribute to feeding and pathogen transmission.
Implications for future tick research
One of the underlying goals of tick research is the development of an anti-tick vaccine, which requires the identification of suitable targets for vaccination. Several antigenic targets have been evaluated including OspA, Bm86, Bm91, longistatin, sialostatin L2, Salp25d and others (Anisuzzaman et al. 2012; Ben Said et al. 2012; Fikrig et al. 1992; Kotsyfakis et al. 2006; Lambertz et al. 2012; Maritz-Olivier et al. 2012; Narasimhan et al. 2007), and although these vaccine candidates do provide partial protection, these targets are not ideal. Several problems have arisen including differences in cross-species protection which may vary significantly or have yet to be evaluated or they lack of sufficient protection for subsequent commercialization. Antioxidant enzymes might be one potential avenue to be further explored in tick vaccination studies.
Interestingly, antioxidant enzymes are the currently being evaluated for their usefulness in developing antiparasitic drugs and as antiparasitic vaccines (Pal and Bandyopadhyay 2012; Prince et al. 2013). A variety of inhibitors have been designed that target glutathione reductase or thioredoxin reductase from Plasmodium falciparum have shown marked antimalarial activity (Pal and Bandyopadhyay 2012), which underscores the central role of the parasitic antioxidant system to its survival. Moreover, there are several examples were antioxidant enzymes have proven to be useful vaccination targets. Protective immunity was induced against Schistosoma mansoni, after a single dose of SmGST recombinant antigen (Grezel et al. 1993). Similarly, mice had some protection against Brugia malayi when immunized a DNA vaccine cocktail which included thioredoxin peroxidase (Anand et al. 2008; Vanam et al. 2009). Interestingly, there was a noted synergistic protective response when mice immunized both Wuchereria bancrofti thioredoxin and thioredoxin peroxidase in an experimental filarial model when challenged with B. malayi larvae (Prince et al. 2013). A similar synergistic protective effect was noted in mice immunized against Helicobacter pylori superoxide dismutase, catalase, and thioredoxin peroxidase (Stent et al. 2012).
The syngergistic effects in vaccination is probably due to the fact that defects in multiple antioxidant enzymes result in additive sensitivity to oxidative stress (Olczak et al. 2002). This work clearly shows that ticks contain a robust secretory antioxidant system, capable of compensating for a single knockdown, given the lack of a significant phenotype. Moving forward, any tick antioxidant antigens should target multiple antioxidant enzymes to more completely affect the vector antioxidant system.
Experimental Procedures
Ticks and animals
Amblyomma maculatum ticks were reared at the University of Southern Mississippi according to established methods (Patrick and Hair 1975). Unfed ticks were maintained at room temperature and 90% relative humidity under 14/10-hour light/dark photoperiod before being placed on sheep (Karim et al. 2002). Briefly, six cells are placed on the back of a shaved sheep, and 40 female and 40 male A. maculatum ticks per cell are placed on the animal’s back (Villarreal et al. 2013). For RNAi studies, 45 ticks are injected with dsRNA, allowed to heal overnight, and forty of the surviving ticks were placed on the animal. Experimental groups and controls were placed on the same animal to eliminate inter-animal variation in feeding success. For transcriptional studies, ticks were allowed to feed, and approximately 5 ticks were removed from the host at regular intervals throughout the blood meal, and the tick tissues were stored in RNAlater (Invitrogen) after dissection. A. maculatum salivary glands were dissected from the ticks within 4 hours of being removed from the host. Adult ticks were fed specifically for this study and all animal studies were performed in accordance with protocols #10042001 and #08110401 approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Southern Mississippi.
Bioinformatics analyses
The full length genes from A. maculatum SelK (GenBank ID: JO843326) and SelM (GenBank ID: JO842653) sequences were obtained from pyrosequencing an A. maculatum salivary gland cDNA library (Karim 2011). Nucleotide sequences were conceptually translated (SelK GenBank ID: AEO34943; SelM GenBank ID: AEO34270), initially aligned using ClustalX2 (Thompson et al. 2002), refined by eye, and graphically presented using Jalview 2.7 (Waterhouse et al. 2009). Phylogenetic relationships were inferred using MEGA 5 (Tamura et al. 2011). Protein secretion signals were identified using SignalP 4.1 (Bendtsen et al. 2004; Petersen et al. 2011). Membrane spanning regions were identified using the DAS transmembrane prediction server (Cserzo et al. 2004; Cserzo et al. 1997).
Tick dissection and saliva collection
Tick tissues were dissected in ice-cold 100 mM MOPS buffer, pH 6.8 containing 20 mM EGTA. After removal, salivary glands and midguts were washed gently in the same ice-cold buffer. The dissected tissues were used immediately after dissection or stored at −80°C in 0.5M PIPES, pH 6.8, containing 20 mM EGTA, protease inhibitor cocktail and 40% glycerol. All other manipulations were carried out at 4°C. Tick saliva was collected as described previously at seven days-post infestation (Ribeiro et al. 1992). Dopamine and theophylline (1 mM each) in 20 mM MOPS buffered saline with 3% DMSO, pH 7.0 were injected as a stimulant for salivation (Needham and Sauer 1979). The saliva was used immediately after collection or stored at −80°C.
RNA preparation and cDNA synthesis
Total RNA was isolated from salivary glands and midguts dissected from unfed and partially-fed adult female ticks using the illustra RNAspin Mini RNA isolation kit (GE Healthcare). The concentration of total RNA was determined spectrophotometrically and samples were aliquoted and stored at −80°C. RNA can spontaneously hydrolyze which can affect the accuracy and reproducibility of results, therefore, cDNA synthesis was then performed (within ~48 hours of RNA isolation). Sample RNA collected was collected seven days post-infestation to compare between SelM and SelK knockdowns with controls (as in Figures 6 and 9) were stored at −80°C until cDNA synthesis could be completed (within 48 hrs). This procedure minimized the impact of differences in cDNA synthesis efficiency from being introduced while minimizing the impact of RNA hydrolysis. Total RNA was reverse-transcribed using Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen) according to the manufacturers’ protocol.
Reverse transcriptase quantitative PCR
Real-time PCR (RT-qPCR) was performed on a Bio-Rad C1000 Thermocycler fitted with the CFX96 Realtime System for fluorescence detection. SYBR Green PCR Mix was obtained from Fermentas and the manufacturer’s instructions were followed. Primer sequences used for QRT-PCR are listed in Table 1 using A. maculatum cDNA. Prior to experimental procedures, the PCR amplicon of each primer set listed in Table 1 was serially diluted ten-fold (2×108–2×101 copies/μL) and used for standard curve preparation. The PCR efficiency of each primer set was 90–110%, the Pearson correlation coefficient exceeded 0.995, and the PCR was linear over at least 5 orders of magnitude. Each 25 μL qPCR reaction consisted of SYBR Green qPCR Master Mix (Fermentas), 50 ng of cDNA, and 150 nM gene specific primers. RT-qPCR were run under the following PCR protocol: 50°C for 3 minutes, 95°C for 10 minutes, followed by 35 cycles of 95°C for 15 seconds, 60°C for 30 seconds, 72°C for 30 seconds. The fluorescence was read after the final extension step. Samples were run in triplicate with no-RT and no-template controls. Expression of each selenoprotein candidate gene and the reference gene, actin, was used to calculate expression values using the Bio-Rad data analysis software using the ΔΔCT. The actin gene is the most suitable reference gene candidate for gene expression in A. maculatum and has previously been validated (Browning et al. 2012). These values were then compared to levels observed in unfed ticks for ease in comparison. A two-fold change in expression and a p-value of 0.05 were the threshold criteria for statistical significance in qRT-PCR assays and were determined using the Bio-Rad CFX manager (version 6).
Table 1.
Primer sequences used in this study for QRT-PCR analysis.
| Gene | GenBank ID | Forward primer (5′-3′) | Reverse primer (5′-3′) | Size (bp) |
|---|---|---|---|---|
| selK | JO843326 | AGTTCCAGCAGGTCATCAGTGTCA | TCCAGGAATAGGGCAGTCCATTGT | 132 |
| selM | JO842653 | ATGATACCTGAATGGCCATCCGCA | TGATCGCGGGTCATCTTCTCCAAA | 171 |
| selN | KC989560 | TTAGTTTGGACACTGTGGACGGGT | AGGCTTCTCTAACAACGGCACTCA | 150 |
| selO | KC989561 | AAGCTCGGCCTTGTGAAGAGAGAA | TACAGCACGACAAGAGCTTGGACA | 190 |
| selS | JO842687 | AGAACAAGTGCACCACAACAGCAG | ATTTCTTGCATCCTTCGACGTGCC | 107 |
| selT | KC989562 | TCTTTGTGTGTGGAGCCATCGAGA | ACCACACCCGCACGTCATTAAAGT | 81 |
| selX | JO845128 | ACCACTCTCCTTGGCCATCATTCA | TGCACTTCCCACAGTACACCTTGA | 108 |
| act | JO842238 | TGGCTCCTTCCACCATGAAGATCA | TAGAAGCACTTGCGGTGCACAATG | 169 |
| gpx | JO843645 | TGCCGCGCTGTCTTTATTATTGGC | AGTTGCACGGAGAACCTCATCGAA | 102 |
| cat | JO843741 | AAAGGACGTCGACATGTTCTGGGA | ACTTGCAGTAGACTGCCTCGTTGT | 173 |
| gshr | JO844062 | ACCTGACCAAGAGCAACGTTGAGA | ATCGCTTGTGATGCCAAACTCTGC | 170 |
| trxR | JO843723 | TGTGACTACACCAACGTGCCTACA | AGTAGCCTGCATCCGTTCCTCTTT | 175 |
Synthesis of selenoprotein-dsRNA and microinjection
Selenoprotein RT-qPCR amplicons were joined to the T7 promoter linker using the Block-iT T7 TOPO kit (Invitrogen). The TOPO linking reaction was used as a template for a PCR reaction containing the T7 PCR primer and a gene-specific primer to produce sense and antisense linear DNA template. After transcription, the sense and antisense ssRNA was purified, annealed, and verified in size by agarose gel electrophoresis. To investigate the role of selenoproteins in tick feeding success in vivo, 50 unfed female ticks were injected with 1000 ng of selM- or selK-dsRNA into the hemocoel using a Hamilton syringe fitted with a 33-gauge needle. After injection of dsRNA, ticks were kept at 37°C overnight under high humidity to confirm tick survival, and then infested on a sheep (Karim and Adamson 2012). Control ticks were injected with 1000 ng GFP-dsRNA, buffer (100 mM Tris-HCl, pH 8.0, 10 mM EDTA, pH 8.0, 1M NaCl) or were mock injected. Ticks were injected with an irrelevant dsRNA (GFP) to control for off-target effects caused by RNAi (Lew-Tabor et al. 2011). Since the GFP gene is not present A. maculatum genome, this should establish if any phenotype observed within the knockdown mutants results from unintended effects resulting from the induction of antiviral machinery.
RNA interference phenotype
Ticks injected with dsRNA were allowed to feed for seven days and were then removed to collect saliva (pooled from 10 ticks) and tissues (5 ticks) for antioxidant assays and prepare RNA to determine efficiency of gene knockdown or the impact on transcriptional gene expression of antioxidant genes (15–20 ticks). Some of the ticks injected with dsRNA were allowed to feed to repletion (10–12 days) and the size of the egg mass and hatching was monitored (5 ticks). Gene knockdown was determined from pooled tick tissues.
Total Antioxidant Capacity
The total antioxidant capacity of pooled tick saliva and tick tissues was determined according to the manufacturer’s protocol using QuantiChrome Antioxidant Assay Kit (BioAssay Systems). Trolox was used as the antioxidant standard. Tissues and saliva were collected from ticks seven days post-infestation. Soluble protein extracts from tick salivary glands and midguts were prepared as previously described (Adamson et al. 2013). Saliva collected from GFP-dsRNA injected ticks was the control.
Acknowledgments
This work was partly supported by Pakistan-US Science and Technology Cooperation Program (US DOS award #PGA-P21049) and NIH NIAID award # AI099919 to SK. We thank Baobin Kang and Khem Raj Budachetri for technical support; the MS-INBRE core facility supported by NIH-NIGMS award #P20RR016476.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- Adamsom SW, Browning RE, Budachetri K, Ribeiro JM, Karim S. Knockdown of Selenocysteine-Specific Elongation Factor in Amblyomma maculatum alters the pathogen burden of Rickettsia parkeri with epigenetic control by the Sin3 Histone Deacetylase Corepressor Complex. PLoS One. 2013;8:e82012. doi: 10.1371/journal.pone.0082012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson SW, Browning RE, Chao CC, Bateman RC, Jr, Ching WM, Karim S. Molecular characterization of tick salivary gland glutaminyl cyclase. Insect Biochem Mol Biol. 2013;43:781–793. doi: 10.1016/j.ibmb.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MV, Kaliraj P. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop. 2008;107:106–12. doi: 10.1016/j.actatropica.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Anisuzzaman, Islam MK, Alim MA, Miyoshi T, Hatta T, Yamaji K, Matsumoto Y, Fujisaki K, Tsuji N. Longistatin is an unconventional serine protease and induces protective immunity against tick infestation. Mol Biochem Parasitol. 2012;182:45–53. doi: 10.1016/j.molbiopara.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Arbogast S, Ferreiro A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid Redox Signal. 2010;12:893–904. doi: 10.1089/ars.2009.2890. [DOI] [PubMed] [Google Scholar]
- Ben Said M, Galai Y, Mhadhbi M, Jedidi M, de la Fuente J, Darghouth MA. Molecular characterization of Bm86 gene orthologs from Hyalomma excavatum, Hyalomma dromedarii and Hyalomma marginatum marginatum and comparison with a vaccine candidate from Hyalomma scupense. Vet Parasitol. 2012;190:230–40. doi: 10.1016/j.vetpar.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bezuidenhout JD. Natural transmission of heartwater. Onderstepoort J Vet Res. 1987;54:349–51. [PubMed] [Google Scholar]
- Brown SJ. Evidence for regurgitation by Amblyomma americanum. Vet Parasitol. 1988;28:335–42. doi: 10.1016/0304-4017(88)90081-7. [DOI] [PubMed] [Google Scholar]
- Browning R, Adamson S, Karim S. Choice of a stable set of reference genes for qRT-PCR analysis in Amblyomma maculatum (Acari: Ixodidae) J Med Entomol. 2012;49:1339–46. doi: 10.1603/me12123. [DOI] [PubMed] [Google Scholar]
- Castets P, Lescure A, Guicheney P, Allamand V. Selenoprotein N in skeletal muscle: from diseases to function. J Mol Med (Berl) 2012;90:1095–107. doi: 10.1007/s00109-012-0896-x. [DOI] [PubMed] [Google Scholar]
- Chapple CE, Guigo R. Relaxation of selective constraints causes independent selenoprotein extinction in insect genomes. PLoS One. 2008;3:e2968. doi: 10.1371/journal.pone.0002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citelli M, Lara FA, da Silva Vaz I, Jr, Oliveira PL. Oxidative stress impairs heme detoxification in the midgut of the cattle tick, Rhipicephalus (Boophilus) microplus. Mol Biochem Parasitol. 2007;151:81–8. doi: 10.1016/j.molbiopara.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Cserzo M, Eisenhaber F, Eisenhaber B, Simon I. TM or not TM: transmembrane protein prediction with low false positive rate using DAS-TMfilter. Bioinformatics. 2004;20:136–7. doi: 10.1093/bioinformatics/btg394. [DOI] [PubMed] [Google Scholar]
- Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–6. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis. 2001;184:1056–64. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- Diaz-Albiter H, Mitford R, Genta FA, Sant’Anna MR, Dillon RJ. Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS One. 2011;6:e17486. doi: 10.1371/journal.pone.0017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Zhou J, Jia Y, Huang K. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys. 2010;502:137–43. doi: 10.1016/j.abb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Ferguson AD, Labunskyy VM, Fomenko DE, Arac D, Chelliah Y, Amezcua CA, Rizo J, Gladyshev VN, Deisenhofer J. NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J Biol Chem. 2006;281:3536–43. doi: 10.1074/jbc.M511386200. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Kantor FS, Flavell RA. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992;60:773–7. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–35. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Grezel D, Capron M, Grzych JM, Fontaine J, Lecocq JP, Capron A. Protective immunity induced in rat schistosomiasis by a single dose of the Sm28GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol. 1993;23:454–60. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- Hirosawa-Takamori M, Chung HR, Jackle H. Conserved selenoprotein synthesis is not critical for oxidative stress defence and the lifespan of Drosophila. EMBO Rep. 2004;5:317–22. doi: 10.1038/sj.embor.7400097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Hoffmann FW, Norton RL, Hashimoto AC, Hoffmann PR. Selenoprotein K is a novel target of m-calpain, and cleavage is regulated by Toll-like receptor-induced calpastatin in macrophages. J Biol Chem. 2011;286:34830–8. doi: 10.1074/jbc.M111.265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Sin JS, Kim MS, Yim SY, Kim YK, Kim CK, Kim BG, Shim SB, Jee SW, Lee SH, Bae CJ, Lee BC, Jang MK, Cho JS, Chae KR. Overexpression of human selenoprotein M differentially regulates the concentrations of antioxidants and H2O2, the activity of antioxidant enzymes, and the composition of white blood cells in a transgenic rat. Int J Mol Med. 2008;21:169–79. [PubMed] [Google Scholar]
- Jurynec MJ, Xia R, Mackrill JJ, Gunther D, Crawford T, Flanigan KM, Abramson JJ, Howard MT, Grunwald DJ. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc Natl Acad Sci U S A. 2008;105:12485–90. doi: 10.1073/pnas.0806015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Adamson SW. RNA Interference in Ticks: A Functional Genomics Tool for the Study of Physiology. In: Jockusch EL, editor. Small RNAs: Their Diversity, Roles, and Practical Uses. Vol. 42. Elsevier; Storrs: 2012. [Google Scholar]
- Karim S, Essenberg RC, Dillwith JW, Tucker JS, Bowman AS, Sauer JR. Identification of SNARE and cell trafficking regulatory proteins in the salivary glands of the lone star tick, Amblyomma americanum (L.) Insect Biochem Mol Biol. 2002;32:1711–21. doi: 10.1016/s0965-1748(02)00111-x. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JMC. A Deep Insight into the Sialotranscriptome of the Gulf Coast Tick, Amblyomma maculatum. PLoS One. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee CB, Means AR. Keeping up with calcium: conference on calcium-binding proteins and calcium function in health and disease. EMBO Rep. 2002;3:823–7. doi: 10.1093/embo-reports/kvf182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan KM, Zivkovic Z, Blouin EF, Naranjo V, Almazan C, Mitra R, de la Fuente J. Silencing of genes involved in Anaplasma marginale-tick interactions affects the pathogen developmental cycle in Dermacentor variabilis. BMC Dev Biol. 2009;9:42. doi: 10.1186/1471-213X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN. Association between the 15-kDa selenoprotein and UDP-glucose:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2001;276:15330–6. doi: 10.1074/jbc.M009861200. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Sa-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281:26298–307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Labunskyy VM, Ferguson AD, Fomenko DE, Chelliah Y, Hatfield DL, Gladyshev VN. A novel cysteine-rich domain of Sep15 mediates the interaction with UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 2005;280:37839–45. doi: 10.1074/jbc.M508685200. [DOI] [PubMed] [Google Scholar]
- Lambertz C, Chongkasikit N, Jittapalapong S, Gauly M. Immune Response of Bos indicus Cattle against the Anti-Tick Antigen Bm91 Derived from Local Rhipicephalus (Boophilus) microplus Ticks and Its Effect on Tick Reproduction under Natural Infestation. J Parasitol Res. 2012;2012:907607. doi: 10.1155/2012/907607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew-Tabor AE, Kurscheid S, Barrero R, Gondro C, Moolhuijzen PM, Rodriguez Valle M, Morgan JA, Covacin C, Bellgard MI. Gene expression evidence for off-target effects caused by RNA interference-mediated gene silencing of Ubiquitin-63E in the cattle tick Rhipicephalus microplus. Int J Parasitol. 2011;41:1001–14. doi: 10.1016/j.ijpara.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Lobanov AV, Hatfield DL, Gladyshev VN. Selenoproteinless animals: selenophosphate synthetase SPS1 functions in a pathway unrelated to selenocysteine biosynthesis. Protein Sci. 2008;17:176–82. doi: 10.1110/ps.073261508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanov AV, Hatfield DL, Gladyshev VN. Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta. 2009;1790:1424–8. doi: 10.1016/j.bbagen.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Koonin EV. A superfamily of archaeal, bacterial, and eukaryotic proteins homologous to animal transglutaminases. Protein Sci. 1999;8:1714–9. doi: 10.1110/ps.8.8.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Mariotti M, Ridge PG, Zhang Y, Lobanov AV, Pringle TH, Guigo R, Hatfield DL, Gladyshev VN. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One. 2012;7:e33066. doi: 10.1371/journal.pone.0033066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz-Olivier C, van Zyl W, Stutzer C. A systematic, functional genomics, and reverse vaccinology approach to the identification of vaccine candidates in the cattle tick, Rhipicephalus microplus. Ticks Tick Borne Dis. 2012;3:179–87. doi: 10.1016/j.ttbdis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Jauron SD, Kurtti TJ. The tick: a different kind of host for human pathogens. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-borne diseases of humans. Vol. 1. ASM Press; Washington D.C: 2005. p. 37. [Google Scholar]
- Narasimhan S, Sukumaran B, Bozdogan U, Thomas V, Liang X, DePonte K, Marcantonio N, Koski RA, Anderson JF, Kantor F, Fikrig E. A tick antioxidant facilitates the Lyme disease agent’s successful migration from the mammalian host to the arthropod vector. Cell Host Microbe. 2007;2:7–18. doi: 10.1016/j.chom.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham GR, Sauer JR. Involvement of calcium and cyclic AMP in controlling ixodid tick salivary fluid secretion. J Parasitol. 1979;65:531–42. [PubMed] [Google Scholar]
- Olczak AA, Olson JW, Maier RJ. Oxidative-stress resistance mutants of Helicobacter pylori. J Bacteriol. 2002;184:3186–93. doi: 10.1128/JB.184.12.3186-3193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–11. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Pal C, Bandyopadhyay U. Redox-active antiparasitic drugs. Antioxid Redox Signal. 2012;17:555–82. doi: 10.1089/ars.2011.4436. [DOI] [PubMed] [Google Scholar]
- Patrick CD, Hair JA. Laboratory rearing procedures and equipment for multi-host ticks (Acarina: Ixodidae) J Med Entomol. 1975;12:389–90. doi: 10.1093/jmedent/12.3.389. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–6. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Prince PR, Madhumathi J, Anugraha G, Jeyaprita PJ, Reddy MV, Kaliraj P. Tandem antioxidant enzymes confer synergistic protective responses in experimental filariasis. J Helminthol. 2013:1–9. doi: 10.1017/S0022149X13000333. [DOI] [PubMed] [Google Scholar]
- Rederstorff M, Castets P, Arbogast S, Laine J, Vassilopoulos S, Beuvin M, Dubourg O, Vignaud A, Ferry A, Krol A, Allamand V, Guicheney P, Ferreiro A, Lescure A. Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy. PLoS One. 2011;6:e23094. doi: 10.1371/journal.pone.0023094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–78. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Evans PM, MacSwain JL, Sauer J. Amblyomma americanum: characterization of salivary prostaglandins E2 and F2 alpha by RP-HPLC/bioassay and gas chromatography-mass spectrometry. Exp Parasitol. 1992;74:112–6. doi: 10.1016/0014-4894(92)90145-z. [DOI] [PubMed] [Google Scholar]
- Sandalova T, Zhong L, Lindqvist Y, Holmgren A, Schneider G. Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc Natl Acad Sci U S A. 2001;98:9533–8. doi: 10.1073/pnas.171178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchedrina VA, Everley RA, Zhang Y, Gygi SP, Hatfield DL, Gladyshev VN. Selenoprotein k binds multiprotein complexes and is involved in the regulation of endoplasmic reticulum homeostasis. J Biol Chem. 2011a;286:42937–48. doi: 10.1074/jbc.M111.310920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchedrina VA, Kabil H, Vorbruggen G, Lee BC, Turanov AA, Hirosawa-Takamori M, Kim HY, Harshman LG, Hatfield DL, Gladyshev VN. Analyses of fruit flies that do not express selenoproteins or express the mouse selenoprotein, methionine sulfoxide reductase B1, reveal a role of selenoproteins in stress resistance. J Biol Chem. 2011b;286:29449–61. doi: 10.1074/jbc.M111.257600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stent A, Every AL, Ng GZ, Chionh YT, Ong LS, Edwards SJ, Sutton P. Helicobacter pylori thiolperoxidase as a protective antigen in single- and multi-component vaccines. Vaccine. 2012;30:7214–20. doi: 10.1016/j.vaccine.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2002;Chapter 2(Unit 2 3) doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- Vanam U, Pandey V, Prabhu PR, Dakshinamurthy G, Reddy MV, Kaliraj P. Evaluation of immunoprophylactic efficacy of Brugia malayi transglutaminase (BmTGA) in single and multiple antigen vaccination with BmALT-2 and BmTPX for human lymphatic filariasis. Am J Trop Med Hyg. 2009;80:319–24. [PubMed] [Google Scholar]
- Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol. 2011;186:2127–37. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal AM, Adamson SW, Browning RE, Budachetri K, Sajid MS, Karim S. Molecular characterization and functional significance of the Vti family of SNARE proteins in tick salivary glands. Insect Biochem Mol Biol. 2013;43:483–493. doi: 10.1016/j.ibmb.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–91. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth EK, Conrad M, Winterer J, Wozny C, Carlson BA, Roth S, Schmitz D, Bornkamm GW, Coppola V, Tessarollo L, Schomburg L, Kohrle J, Hatfield DL, Schweizer U. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24:844–52. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang Y, Liu H, Yang H, Ma D, Li J, Li D, Lai R, Yu H. Two immunoregulatory peptides with antioxidant activity from tick salivary glands. J Biol Chem. 2010;285:16606–13. doi: 10.1074/jbc.M109.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]