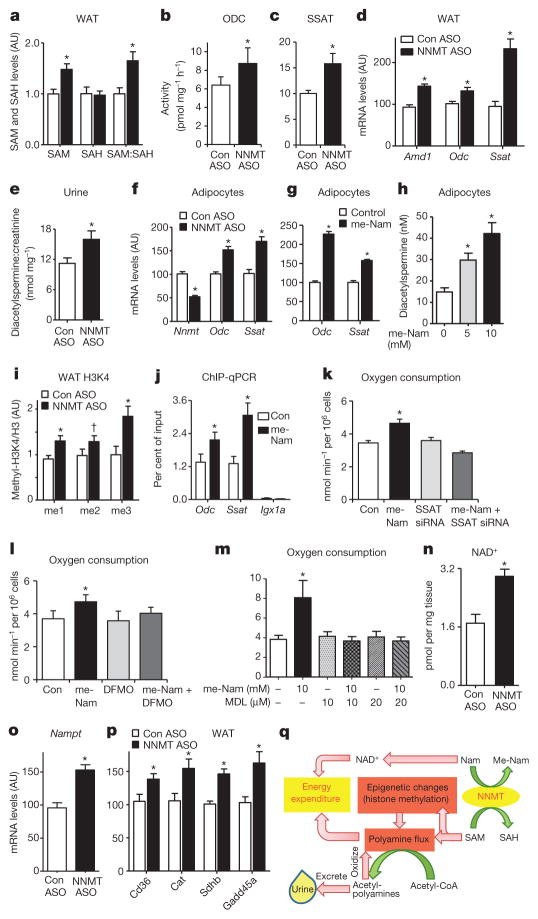
Figure 4. NNMT regulates SAM and NAD+ pathways in adipose tissue.
a, Adipose S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH) and SAM:SAH ratio measured by LC–MS/MS (n =8 for control-ASO-treated mice; n =12 for Nnmt-ASO-treated mice). b, c, ODC (b) and SSAT (c) activity (n =10 per group). d, Amd1, Odc and Ssat mRNA expression in adipose tissue of Nnmt-ASO- and control-ASO-treated mice (n =12 per group). e, Urinary diacetylspermine:creatinine ratio (n =22 for control-ASO-treated mice; n =29 for Nnmt-ASO-treated mice). f, g, Odc and Ssat mRNA levels in 3T3-L1 adipocytes with Nnmt knockdown (n =9 per group) (f) and N1-methylnicotinamide (me-Nam) (g) treatment (n =6 per group). h, Diacetylspermine secretion from 3T3-L1 adipocytes treated with me-Nam (n =10 per group). i, Expression of mono-, di- and tri-methylation of lysine histone 3 (H3K4) normalized to total H3 levels in adipose tissue (n =8 per group). j, H3K4me2 occupancy on Odc, Ssat genes and an open reading frame free region (Igx1a) as a negative control in adipocytes measured by ChIP-qPCR (n =12 per group). k–m, Oxygen consumption in adipocytes transfected with control or Ssat siRNA, and treated with or without 10 mM me-Nam (n =6 per group) (k); treated with 10 mM me-Nam with or without 5 mM difluoromethylornithine (DFMO), a specific ODC inhibitor (n =6 per group) (l); or treated with 10 mM me-Nam with or without MDL72527, a specific PAO inhibitor (n =6 per group) (m). n, NAD+ levels (n =7 Con-ASO; n =12 Nnmt ASO). o, mRNA levels of nicotinamide phosphoribosyltransferase (Nampt) (n =11 per group). p, mRNA levels of SIRT1 target genes: Cd36, catalase (Cat), succinate dehydrogenase B (SdhB) and growth arrest and DNA-damage-inducible protein (Gadd45a) in adipose tissue of NNMT-ASO- and control-ASO treated mice (n =11 per group). Error bars, ± s.e.m., *P <0.05, †P =0.06. q, Model of NNMT-regulated energy expenditure in adipocytes. NNMT methylates nicotinamide (Nam), a precursor of NAD+, using SAM as a methyl donor. SAM regulates polyamine flux by providing substrates and modulating histone methylation. Polyamine flux utilizes acetyl-CoA to generate acetyl-polyamines, which are oxidized or excreted in the urine. Taken together, this results in adipose metabolic substrate consumption and loss coupled with systemic alteration of energy expenditure.