Abstract
The aberrant pyramidal tract is the collateral pathway of the pyramidal tract through the medial lemniscus in the brainstem. A 21-year-old man presented with right hemiparesis due to a traumatic intracerebral hemorrhage in the left corona radiata. His motor function recovered almost to the normal state at 10 months after onset. Through diffusion tensor tractography, the pyramidal tract in the affected (left) hemisphere showed discontinuation at the pontine level at 13 months after onset. An aberrant pyramidal tract was observed, which originated from the primary motor cortex and the supplementary motor area and descended through the corona radiata, then through the posterior limb of the internal capsule and the medial lemniscus pathway from the midbrain to the pons, finally entered into the pyramidal tract area at the pontomedullary junction. It suggests that the motor functions of the right extremities in this patient had recovered by this aberrant pyramidal tract.
Keywords: neural regeneration, neuroimaging, diffusion tensor imaging, diffusion tensor tractography, transcranial magnetic stimulation, pyramidal tract, aberrant pyramidal tract, motor paralysis, motor recovery, traumatic brain injury, head trauma, intracerebral hemorrhage, grant-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) The aberrant pyramidal tract is the collateral pathway of the pyramidal tract through the medial lemniscus in the brainstem.
(2) Through diffusion tensor tractography, an aberrant pyramidal tract was observed in a patient with traumatic intracerebral hemorrhage in the left corona radiata using diffusion tensor tractography.
(3) The main motor functions of the right extremities in this patient appeared to have recovered by this aberrant pyramidal tract.
INTRODUCTION
Detailed information about motor recovery is essential for brain rehabilitation because it enables us to determine appropriate and scientific rehabilitative strategies. However, motor recovery in traumatic brain injury is little studied compared to other neurologic diseases involving the brain such as stroke[1,2]. Only several studies have been reported on the mechanism of traumatic brain injury recovery[3,4,5,6,7].
The pyramidal tract is a major neuronal pathway for mediation of voluntary movements in the human brain[8,9,10,11]. The aberrant pyramidal tract refers to the of the pyramidal tract through the medial lemniscus in the brainstem[12,13,14,15]. Recently, a motor recovery mechanism in stroke has been suggested, but very little is known about traumatic brain injury[16,17,18,19]. In the current study, we report on a patient with traumatic brain injury, whose motor function appeared to have recovered via an aberrant pyramidal tract as demonstrated by diffusion tensor tractography.
CASE REPORT
A 21-year-old right-handed man was admitted by Yeungnam University Hospital for evaluation of motor function and rehabilitation. The patient presented with severe motor paralysis in the right extremity due to the traumatic intracerebral hemorrhage in the left corona radiate. T2-weighted magnetic resonance images taken at 3 months showed encephalomalactic lesions in the left anterior to middle corona radiata and basal ganglia (Figure 1A).
Figure 1.
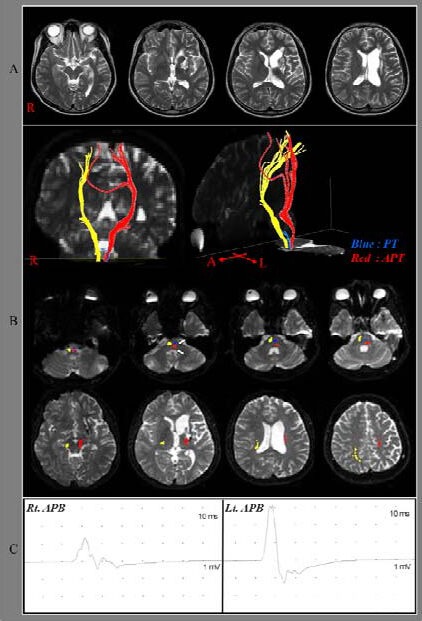
T2-weighted brain images and results of diffusion tensor tractography for the pyramidal track in a patient with traumatic brain injury.
(A) T2-weighted brain images (3 months after onset) showing leukomalactic lesions in the left anterior to middle corona radiata and basal ganglia.
(B) Diffusion tensor tractography (13 months after onset). The pyramidal tract of the unaffected (right, yellow color) hemisphere originates from the primary sensori-motor cortex and descends through the known pyramidal track pathway. There was no significant injury of pyramidal track in the unaffected hemisphere. However, the left pyramidal track was disrupted at the upper pons and an aberrant pyramidal track, which descends through the medial lemniscus pathway from the midbrain to the pons, and then enters into the pyramidal track area at the pontomedullary junction.
(C) Transcranial magnetic stimulation (13 months after onset). Motor-evoked potentials were evoked from both hemispheres.
R, Rt: Right; Lt: left; APT: aberrant pyramidal tract; PT: pyramidal tract; APB: abductor pollicis brevis.
Clinical evaluation
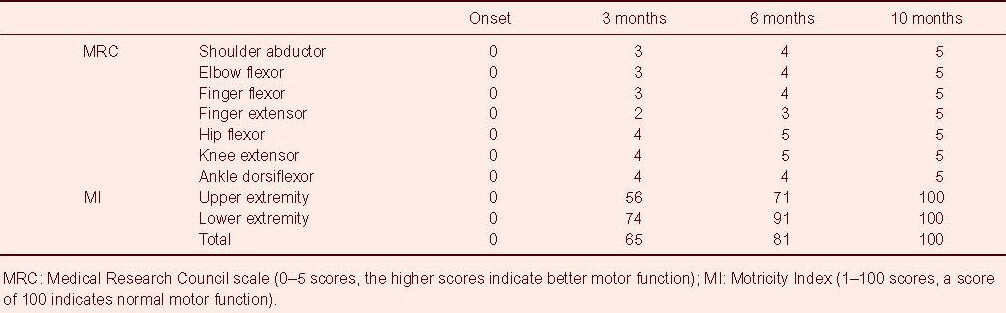
The Motricity Index and Medical Research Council scale, which were measures of the integrity of the motor function, were used to determine motor function at onset time, and 3, 6 and 10 months after onset[20]. Morticity Index has two different evaluation scores in hand and other joints (shoulder, elbow, hip, knee and ankle); hand prehension: 0 (non-movement), 33 (beginning of prehension), 56 (grips cube, without gravity), 65 (holds cube, against gravity), 77 (grips against pull, but weaker than other side), and 100 (normal); other joints: 0 (no movement), 28 (palpable contraction), 42 (movement without gravity), 56 (movement against gravity), 74 (movement against resistance, but weaker than normal), and 100 (normal). In addition, six categories are included in the Medical Research Council scale: 0, no contraction; 1, palpable contraction, but no visible movement; 2, movement without gravity; 3, movement against gravity; 4, movement against a resistance lower than the resistance overcome by the healthy side; 5, movement against a resistance equal to the maximum resistance overcome by the healthy side. The patient presented with severe weakness of the right upper and lower extremities at the onset of traumatic intracerebral hemorrhage. However, the motor function of his right extremities had recovered to the level that he was able to extend the affected fingers against gravity at 6 months (Table 1). Ten months after onset, the patient was able to perform fine motor activities, as well as walk with a normal gait.
Table 1.
Changes of the motor function in the included patient
Diffusion tensor tractography
Diffusion tensor imaging was performed at 13 months after onset using a sensitivity encoding head coil on a 1.5 T Philips Gyroscan Intera (Hoffman-LaRoche, Best, Netherlands) with single-shot echo-planar imaging. A total of 60 contiguous slices (matrix = 128 × 128, field of view = 221 × 221 mm2, repetition time/echo time = 10 726/76 ms, b = 1 000 mm2/s, number of excitations = 1, thickness = 2.3 mm) were acquired for each of the 32 noncollinear diffusion-sensitizing gradients. Eddy current image distortions and motion artifacts were corrected using affine multi-scale two-dimensional registration, which was performed using the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). Diffusion tensor imaging-Studio software (CMRM, Johns Hopkins Medical Institute, USA) was used for evaluation of the pyramidal track[21]. For fiber tracking of the pyramidal track, a region of interest was drawn in the pyramidal track portion of the pontomedullary junction, and target region of interest was placed on the lateral half of the midpons. Fiber tracking was performed with fractional anisotropy at a threshold > 0.2 and direction threshold < 60°[22].
The pyramidal tracks of the unaffected (right) hemisphere originated from the primary sensori-motor cortex and descended through the known pyramidal track pathway on diffusion tensor tractography. However, the left pyramidal track showed discontinuation at the pons. In addition, we observed an aberrant pyramidal track in the left hemisphere, which originated from the primary motor cortex and the supplementary motor area and descended through the corona radiata, the posterior limb of the internal capsule and the medial lemniscus pathway from the midbrain to the pons, and finally entered into the pyramidal track area at the pontomedullary junction (Figure 1B).
Transcranial magnetic stimulation
Transcranial magnetic stimulation was performed at 13 months after onset using a Magstim Novametrix 200 magnetic stimulator with a 9 cm mean diameter circular coil (Novametrix Medical Systems Inc., Wallingford, CT, USA). Cortical stimulation was performed with the coil held tangentially over the vertex. The left hemisphere was stimulated by a counterclockwise current, whereas the right hemisphere was stimulated by a clockwise current. Motor-evoked potentials were obtained from both abductor pollicis brevis muscles in the relaxed state. The excitatory threshold was defined as the minimum stimulus required to elicit a motor-evoked potential with a peak-to-peak amplitude of 50 µV or greater in 2 out of 4 attempts. Stimulation intensity was set at the excitatory threshold plus 20% of the maximum stimulator output. Transcranial magnetic stimulation at 13 months showed that motor-evoked potentials were evoked from both hemispheres (right abductor pollicis brevis muscle–latency: 24.0 ms, amplitude: 1 700 µV, excitatory threshold: 80%; left abductor pollicis brevis muscle–latency: 21.5 ms, amplitude: 4 400 µV, excitatory threshold: 50%; Figure 1C).
DISCUSSION
A recent diffusion tensor tractography study has demonstrated that aberrant pyramidal tract was present in 18.4% of the hemispheres of normal subjects[12]. In our study, we found an aberrant pyramidal tract in the affected hemisphere of a patient with traumatic brain injury affected by pyramidal track injury. The main motor functions of the right extremities in this patient appeared to have recovered by this aberrant pyramidal tract for the following reasons. Brain MRI revealed encephalomalactic lesions in the anterior to middle corona radiata, which appeared to include the pyramidal track[23,24]. Through 13-month diffusion tensor tractography, the affected pyramidal track was discontinued at pontine level, even after the FA threshold was lowered to 0.1. We observed an aberrant pyramidal tract in the left hemisphere, which descended through the medial lemniscus pathway from the midbrain to the pons, and then entered into the pyramidal track area at the pontomedullary junction. The motor-evoked potential on the right abductor pollicis brevis that showed mildly delayed latency could be additional evidence for the aberrant pyramidal tract[4,25].
Since the introduction of diffusion tensor imaging, a few studies have suggested that the aberrant pyramidal tract may contribute to motor recovery in stroke[16,17,18,19]. In 2009, Jang[26] reported a patient whose motor function appeared to have recovered via an aberrant pyramidal tract following a pontine infarct located in the pyramidal track area. In 2010, Lindenberg et al[17] demonstrated that patients with alternate motor fibers in the brainstem showed better motor outcome among 35 patients with middle cerebral artery infarcts. However, they did not clarify that the alternate motor fibers were an aberrant pyramidal tract. Motor recovery via the aberrant pyramidal tract was recently reported in two patients with midbrain infarct or corona radiata[18,19]. Therefore, to the best of our knowledge, this is the first study to report motor recovery via aberrant pyramidal tract in traumatic brain injury.
In conclusion, we reported on a patient whose main motor function of the affected extremities appeared to have recovered by an aberrant pyramidal tract in the brainstem. Our findings have important implications for elucidating the motor recovery mechanism in traumatic brain injury because they may suggest additional motor recovery mechanisms. However, limitations of diffusion tensor imaging should be considered in the interpretation of the results[26,27,28]. The fiber tracking technique is operator-dependent and diffusion tensor imaging may underestimate fiber tracts. Regions of fiber complexity and crossing can obscure the underlying fiber architecture. In addition, this study is limited because it is a case report. Therefore, additional complementary studies involving larger case numbers are warranted.
Footnotes
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012R1A1A4A01001873.
Conflicts of interest: None declared.
(Edited by Song LP)
REFERENCES
- [1].Jang SH. Review of motor recovery in patients with traumatic brain injury. NeuroRehabilitation. 2009;24:349–353. doi: 10.3233/NRE-2009-0489. [DOI] [PubMed] [Google Scholar]
- [2].Jang SH. A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. NeuroRehabilitation. 2011;28:345–352. doi: 10.3233/NRE-2011-0662. [DOI] [PubMed] [Google Scholar]
- [3].Han BS, Kim SH, Kim OL, et al. Recovery of corticospinal tract with diffuse axonal injury: a diffusion tensor image study. NeuroRehabilitation. 2007;22:151–155. [PubMed] [Google Scholar]
- [4].Jang SH, Cho SH, Kim YH, et al. Motor recovery mechanism of diffuse axonal injury: a combined study of transcranial magnetic stimulation and functional MRI. Restor Neurol Neurosci. 2005;23:51–56. [PubMed] [Google Scholar]
- [5].Skoglund TS, Nilsson D, Ljungberg M, et al. Long-term follow-up of a patient with traumatic brain injury using diffusion tensor imaging. Acta Radiol. 2008;49:98–100. doi: 10.1080/02841850701561372. [DOI] [PubMed] [Google Scholar]
- [6].Jang SH, Han BS, Chang Y, et al. Functional MRI evidence for motor cortex reorganization adjacent to a lesion in a primary motor cortex. Am J Phys Med Rehabil. 2002;81:844–847. doi: 10.1097/00002060-200211000-00007. [DOI] [PubMed] [Google Scholar]
- [7].Kim DG, Kim SH, Kim OL, et al. Long-term recovery of motor function in a quadriplegic patient with diffuse axonal injury and traumatic hemorrhage: a case report. NeuroRehabilitation. 2009;25:117–122. doi: 10.3233/NRE-2009-0506. [DOI] [PubMed] [Google Scholar]
- [8].Davidoff RA. The pyramidal tract. Neurology. 1990;40:332–339. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- [9].Nathan PW, Smith MC. Long descending tracts in man. I. Review of present knowledge. Brain. 1955;78:248–303. doi: 10.1093/brain/78.2.248. [DOI] [PubMed] [Google Scholar]
- [10].Nyberg-Hansen R, Rinvik E. Some comments on the pyramidal tract, with special reference to its individual variations in man. Acta Neurol Scand. 1963;39:1–30. [Google Scholar]
- [11].Crosby EC. New York: Macmillan; 1962. Correlative Anatomy of the Nervous System. [Google Scholar]
- [12].Hong JH, Son SM, Byun WM, et al. Aberrant pyramidal tract in medial lemniscus of brainstem in the human brain. Neuroreport. 2009;20:695–697. doi: 10.1097/wnr.0b013e32832a5c86. [DOI] [PubMed] [Google Scholar]
- [13].Yamamoto T. Aberrant pyramidal tract: A clinicopathological review. Neurol Med Chir (Tokyo) 1995;43:306–312. [Google Scholar]
- [14].Yamashita M, Yamamoto T. Aberrant pyramidal tract in the medial lemniscus of the human brainstem: normal distribution and pathological changes. Eur Neurol. 2001;45:75–82. doi: 10.1159/000052099. [DOI] [PubMed] [Google Scholar]
- [15].Yamamoto T. Aberrant pyramidal tract: a study with Sudan III stain. No To Shinkei. 1989;41:777–780. [PubMed] [Google Scholar]
- [16].Jang SH. Aberrant pyramidal tract in the medial lemniscus of the brainstem in a patient with a pontine infarct: diffusion tensor tractography study. J Neurol Neurosurg Psychiatry. 2009;80:243–244. doi: 10.1136/jnnp.2008.146571. [DOI] [PubMed] [Google Scholar]
- [17].Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yeo SS, Jang SH. Motor recovery via aberrant pyramidal tract in a patient with a cerebral peduncle infarct. Neural Regen Res. 2011;6:1023–1026. doi: 10.3969/j.issn.1673-5374.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hong JH, Jang SH. Aberrant pyramidal tract in a patient with corona radiata infarct: A diffusion tensor tractography study. Neural Regen Res. 2011;6:1027–1030. doi: 10.3969/j.issn.1673-5374.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19:382–389. doi: 10.1159/000115178. [DOI] [PubMed] [Google Scholar]
- [21].Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [22].Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci. 2004;3:11–17. doi: 10.2463/mrms.3.11. [DOI] [PubMed] [Google Scholar]
- [23].Han BS, Hong JH, Hong C, et al. Location of the corticospinal tract at the corona radiata in human brain. Brain Res. 2010;1326:75–80. doi: 10.1016/j.brainres.2010.02.050. [DOI] [PubMed] [Google Scholar]
- [24].Jang SH. A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation. 2009;24:279–283. doi: 10.3233/NRE-2009-0479. [DOI] [PubMed] [Google Scholar]
- [25].Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- [26].Jang SH. Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med J. 2011;52:553–557. doi: 10.3349/ymj.2011.52.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005;25:53–65. doi: 10.1148/rg.251045085. [DOI] [PubMed] [Google Scholar]
- [28].Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360:893–902. doi: 10.1098/rstb.2005.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]


