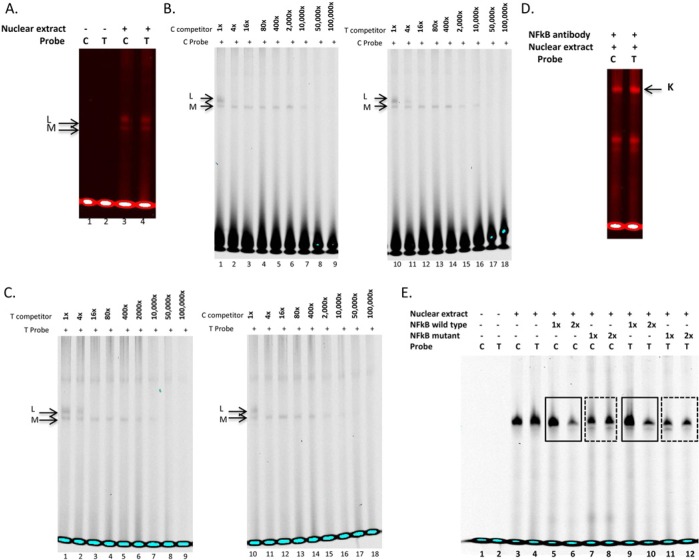
Figure 3.
Electrophoretic mobility shift assays with TIMP2 rs8179096 variant. (A) Double-stranded, infrared-labeled C and T probes incubated in the absence (lanes 1, 2) or presence of cell nuclear extract (lanes 3, 4). (B) Competition assay with double-stranded, infrared labeled C probe incubated in the presence of cell nuclear extract and 1-, 4-, 16-, 80-, 400-, 2,000-, 10,000-, 50,000-, and 100,000-fold molar excess of unlabeled C (lanes 1-9) and T probes (lanes 10-18). (C) Competition assay with double-stranded, infrared-labeled T probe incubated in the presence of cell nuclear extract and 1-, 4-, 16-, 80-, 400-, 2,000-, 10,000-, 50,000-, and 100,000-fold molar excess of unlabeled T (lanes 1-9) and C probes (lanes 10-18). (D) Supershift assays were performed incubating double-stranded, infrared-labeled C and T probes with cell nuclear extract plus NFκB antibody (Complex K). (E) EMSA for verification of NFκB binding to rs8179096. Double-stranded, infrared-labeled C and T probes were incubated in the absence (lanes 1, 2) or presence of cell nuclear extract (lanes 3-12). One- or two-fold molar excess (in comparison with probe concentration) of NFκB consensus unlabeled oligonucleotide was added in lanes 5, 6, 9, and 10 (solid frames), whereas mutant NFκB consensus unlabeled oligonucleotide was added in lanes 7, 8, 11, and 12 (dotted frames). No bands were observed in isotype control lanes, indicating that the supershift bands reflected NFκB-probe interaction.