Abstract
Fluorescent neuronal tracers should not be toxic to the nervous system when used in long-term labeling. Previous studies have addressed tracer toxicity, but whether tracers injected into an intact nerve result in functional impairment remains to be elucidated. In the present study, we examined the functions of motor, sensory and autonomic nerves following the application of 5% Fluoro-Gold, 4% True Blue and 10% Fluoro-Ruby (5 μL) to rat tibial nerves via pressure injection. A set of evaluation methods including walking track analysis, plantar test and laser Doppler perfusion imaging was used to determine the action of the fluorescent neuronal tracers. Additionally, nerve pathology and ratio of muscle wet weight were also observed. Results showed that injection of Fluoro-Gold significantly resulted in loss of motor nerve function, lower plantar sensibility, increasing blood flow volume and higher neurogenic vasodilatation. Myelinated nerve fiber degeneration, unclear boundaries in nerve fibers and high retrograde labeling efficacy were observed in the Fluoro-Gold group. The True Blue group also showed obvious neurogenic vasodilatation, but less severe loss of motor function and degeneration, and fewer labeled motor neurons were found compared with the Fluoro-Gold group. No anomalies of motor and sensory nerve function and no myelinated nerve fiber degeneration were observed in the Fluoro-Ruby group. Experimental findings indicate that Fluoro-Gold tracing could lead to significant functional impairment of motor, sensory and autonomic nerves, while functional impairment was less severe following True Blue tracing. Fluoro-Ruby injection appears to have no effect on neurological function.
Keywords: neural regeneration, peripheral nerve injury, neuronal tracing, tracer toxicity, neurological function, Fluoro-Gold, True Blue, Fluoro-Ruby, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Neuronal tracers are widely used in studies addressing neuroanatomy and neural regeneration. True Blue is potentially suitable for long-term neuronal labeling, while Fluoro-Ruby and Fluoro-Gold only allow short-term labeling. The tracer used needs to be non-toxic or of low neural toxicity for long-term labeling. Although the toxicity of neural tracers has been reported to be pathological, it remains unclear whether and how neural function is affected when an intact nerve is exposed to these tracers.
-
(2)
After the tibial nerve was injected with neural tracers, neurological function was evaluated through several indexes.
-
(3)
Fluoro-Gold tracing could lead to significant functional impairment of motor, sensory and autonomic nerves, while functional impairment was less severe following True Blue tracing. Fluoro-Ruby injection did not affect neurological function.
INTRODUCTION
Neuronal tracing is a common technique used not only in neuroanatomical studies to identify neurocircuitry or axonal projections[1,2,3,4,5], but also in neural regeneration research for the evaluation of re-innervation or reconstruction of fiber tracts after spinal cord injury[6,7,8,9,10]. Among a variety of neuronal tracers used in tract tracing, fluorescent tracers are particularly useful because they allow direct visualization of labeled neurons[6,11,12].
Theoretically, an optimal fluorescent neuronal tracer should possess properties such as neuron-specific labeling, high efficacy, minimal fading, short survival time, and no/low toxicity[13]. Unfortunately, few commercially available neuronal tracers meet all these requirements. For example, Fluoro-Gold is recognized as a potent retrograde tracer with high labeling efficacy and good resistance during tissue preparation, but it has been found to potentially result in neural cell death[13,14].
For most neuroanatomical studies, animals are allowed to survive for a relatively short period, i.e. several days, post-experiment and neuronal toxicity elicited by the tracers themselves is often neglected[3,6,15,16]. However, for studies in which animals should be allowed to survive for a longer period of time, e.g. several weeks to months after tracer application, the tracer used needs to be non-toxic or of low neural toxicity and thus lead to little functional impairment[11,17]. A typical example is the sequential retrograde tracing for assessing regeneration accuracy of peripheral nerves.
In these studies, a tracer (the first tracer) labels the neuronal pool of a specific nerve/region and another tracer (the second tracer) labels the neurons which regenerated and re-innervated the same nerve/region[11]. In this case, the first tracer should be capable of allowing long-term labeling, and should neither lead to functional loss nor impair neural regeneration capacity[14].
Previous studies have shown that True Blue, Fast Blue, Diamidino Yellow and DiI are all potentially suitable for long-term neuronal labeling while Fluoro-Ruby and Fluoro-Gold are suitably used as the second rather than the first tracer because of rapid fading in vivo[11,18,19]. However, it remains unclear whether and how neural function is affected when an intact nerve is exposed to these tracers. This is a particularly important issue regarding the selection of the first tracer in sequential retrograde tracing for the assessment of regeneration accuracy.
In the present study, we examined the neuronal toxicity of True Blue, Fluoro-Ruby and Fluoro-Gold by evaluating the impairment of motor, sensory and autonomic nerve functions, and observed nerve fiber degeneration and labeling efficacy, using a rat model of tibial nerve injection.
RESULTS
Quantitative analysis of experimental animals
Sixty Sprague-Dawley rats were randomly divided into six groups: the sham surgery group (only left tibial nerve was exposed), tibial nerve transection group (left tibial nerve was completely transected, serving as the controls of denervation), Fluoro-Gold group, True Blue group, Fluoro-Ruby group, and the saline group, with 10 rats in each group. In the Fluoro-Gold, True Blue, Fluoro-Ruby, and saline groups, the tibial nerve was not damaged and received injection of 5% Fluoro-Gold, 4% True Blue, 10% Fluoro-Ruby and saline, respectively.
All 60 rats were involved in the final analysis with no loss.
Neurogenic vasodilatation following tracer injection
Laser Doppler perfusion imaging showed that immediately after injection of Fluoro-Gold solution or True Blue suspension into the tibial nerve, a dramatic increase in skin perfusion was observed in the ipsilateral hind paw, similar to a pattern exhibited after tibial nerve transection. No obvious dermal perfusion increase was found after injection with Fluoro-Ruby solution or saline (Figure 1).
Figure 1.
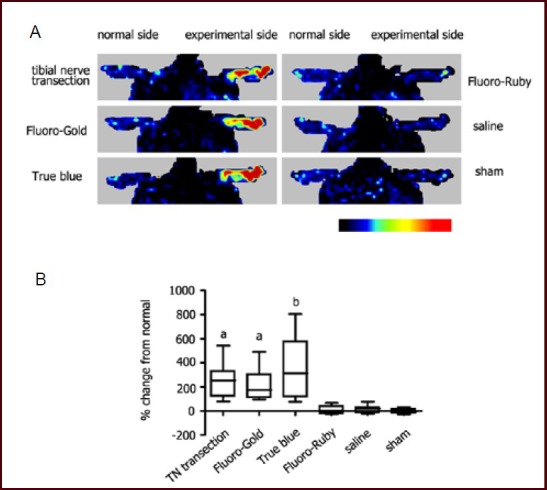
Neurogenic vasodilatation in response to tracer injection.
(A) Representative laser Doppler perfusion images showing alterations of blood perfusion in the foot immediately after tracer exposure. Like nerve transection injury, exposure of the left tibial nerve to either Fluoro-Gold or True Blue resulted in a prompt cutaneous vasodilatation in the left hind paw, but no apparent vasodilatation was observed after injection with Fluoro-Ruby or saline.
(B) Box chart showing quantification data from laser Doppler perfusion images. Data are expressed as percentiles (n = 10, Kruskal-Wallis test, Dunn's multiple comparison test). aP < 0.01, bP < 0.001, vs. Fluoro-Ruby group, saline group and sham surgery (sham) group. TN: Tibial nerve.
This evidence indicated that exposure of an intact nerve to Fluoro-Gold or True Blue, but not Fluoro-Ruby, resulted in neurogenic vasodilatation.
Sensory nerve function following tracer injection
Plantar analgesia tests indicated that only the Fluoro-Gold group showed significantly prolonged withdrawal latency on the tracer injection side compared with other groups at 5 days after tracer injection (Figure 2), while no statistical significance was observed among groups for the contralateral normal side.
Figure 2.
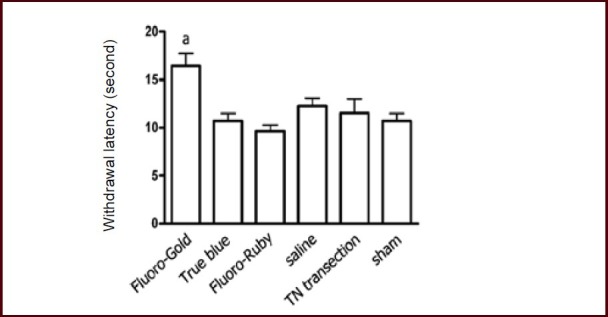
Plantar tests at 5 days after tracer injection.
Bar chart showing withdrawal latency in middle plantar skin on the experimental side.
Data are expressed as mean ± SEM (n = 10), and one-way analysis of variance with Student-Newman-Keuls post hoc test was used for data analysis. aP < 0.01, vs. any of the other groups. TN: Tibial nerve; sham: sham surgery.
Motor nerve function and muscle atrophy following tracer injection
To assess the effect of tracer exposure on motor nerve function, walking track analysis was performed at 3 and 14 days after injection of tracers, and tibial functional index was calculated. Fluoro-Ruby did not seem to hinder motor nerve function at both time points, with similar tibial function index to saline and sham surgery groups. Fluoro-Gold group showed complete loss of function, which was comparable to tibial nerve transection (Figures 3A, B). However, the True Blue group showed partial loss of function, with tibial function index significantly lower than the Fluoro-Ruby group and higher than the Fluoro-Gold group (Figures 3A, B).
Figure 3.
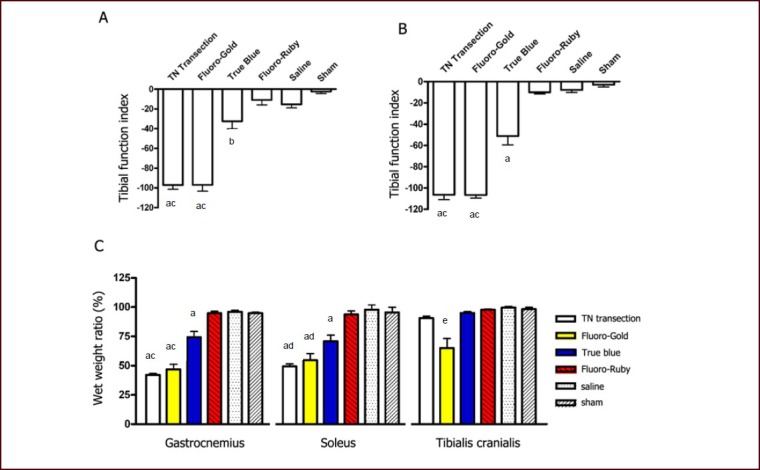
Motor impairment and muscle atrophy in response to tracer injection into the tibial nerve.
(A, B) Bar charts showing tibial function index at 3 and 14 days after tracer injection. No statistical significance was found among Fluoro-Gold, saline and sham surgery groups, nor between Fluoro-Gold and tibial nerve transection groups.
(C) Bar chart showing wet weight ratio (experimental side/contralateral normal side) for gastrocnemius, soleus and tibialis cranialis muscles at 14 days after tracer injection. The wet weight ratio of the tibialis cranialis muscle in the Fluoro-Gold group was significantly lower than any other group. The True Blue group showed less severe atrophy in the gastrocnemius and soleus muscles compared with the Fluoro-Gold group.
Data were expressed as mean ± SEM (n = 10), and one-way analysis of variance with Student-Newman-Keuls post hoc test was used for data analysis. aP < 0.001, bP < 0.05, vs. Fluoro-Ruby, saline and sham surgery groups (A–C). cP < 0.001, dP < 0.01, vs. True Blue group (A–C). eP < 0.001, vs. any of the other groups (C).
TN: Tibial nerve; sham: sham surgery.
At 14 days after tracer exposure, a significantly smaller muscle mass (sign of muscular atrophy) was observed in the gastrocnemius and soleus muscles of the Fluoro-Gold, True Blue and tibial nerve transection groups. The analysis of wet weight ratio revealed a 25% weight loss in the gastrocnemius and soleus muscles for the True Blue group, but a 50% loss for the Fluoro-Gold group. Additionally, unlike tibial nerve transection, the Fluoro-Gold group showed 11–65% (mean 35%) weight loss in the tibialis cranialis muscle (Figure 3C).
Degeneration of nerve fibers and labeling efficacy following tracer injection
Fluoro-Ruby injection into the intact nerve exhibited very few degenerated myelinated axons distal to the injection site at 14 days after tracer injection, as demonstrated by toluidine blue staining of the semi-thin transverse sections. The Fluoro-Gold and True Blue groups showed obvious degeneration (Figure 4A). The majority of myelinated axons were seen to degenerate in the True Blue group with a small fraction remaining intact, while nearly all the myelinated axons were degenerated in the Fluoro-Gold group, leaving numerous condensed degeneration particles (Figures 4A, B). Additionally, the nerve fiber profiles seemed obscure in the Fluoro-Gold group (Figure 4A). All myelinated axons were observed to degenerate, at varying stages, in the tibial nerve transection group, but the profiles of individual myelinated fibers remained clear (Figure 4A).
Figure 4.
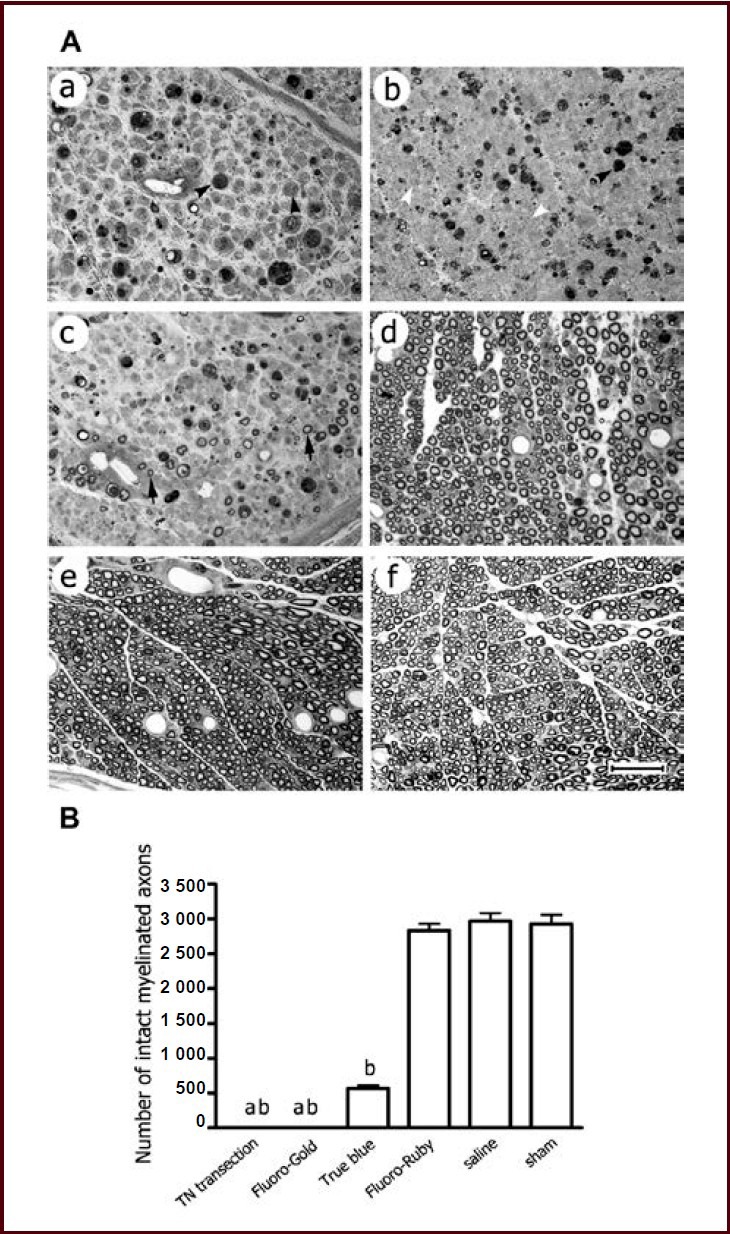
Degeneration of myelinated nerve fibers within the tibial nerve distal to the site of tracer injection at 14 days after tracer injection.
(A) Representative photomicrographs showing semi-thin transverse section of the tibial nerve stained with toluidine blue. The tibial nerve transection, Fluoro-Gold, True Blue, Fluoro-Ruby, saline and sham surgery (sham) groups (a–f) are shown alphabetically. Nearly all the myelinated nerve fibers were seen to be degenerated in the Fluoro-Gold group (b), the majority in the True Blue group (c), but very few in the Fluoro-Ruby and saline groups (d, e). The profiles of individual myelinated nerve fibers seemed obscure in the Fluoro-Gold group, which was in contrast to the tibial nerve transection group where profiles of degenerating fibers remained clear (a). Scale bar: 50 μm.
(B) Bar graph showing counts of intact myelinated axons. Data were expressed as mean ± SEM, and one-way analysis of variance with Student-Newman-Keuls post hoc test was used for data analysis (n = 5). aP < 0.01, vs. True Blue group; bP < 0.001, vs. Fluoro-Ruby, saline and sham groups. TN: Tibial nerve.
At 14 days after tracer injection, over 1 000 motor neurons in the spinal cord were found to be retrogradely labeled in the Fluoro-Gold group. Sixty percent of motor neurons in the True Blue group were labeled compared with the Fluoro-Gold group (Figure 5). Less than 100 labeled motor neurons were observed in the Fluoro-Ruby group (Figure 5).
Figure 5.
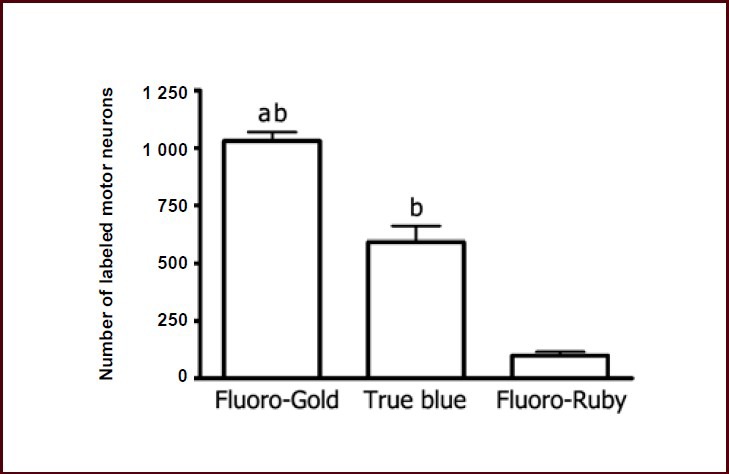
Number of labeled motor neurons in the ventral horn of the spinal cord at 14 days after tracer injection.
Data were expressed as mean ± SEM, and one-way analysis of variance with Student-Newman-Keuls post hoc test was used for data analysis (n = 5). aP < 0.001, vs. True Blue group; bP < 0.001, vs. Fluoro-Ruby group.
DISCUSSION
In this study, motor, sensory and autonomic nerve function was evaluated following the injection of tracers into the tibial nerve, and the results demonstrated that the function of an intact nerve might be impaired after the nerve was injected with Fluoro-Gold or True Blue, but not Fluoro-Ruby.
It has been well documented that walking track analysis and wet weight ratio are sensitive indices for motor nerve innervation and function[20,21,22,23]. In this study, unlike Fluoro-Ruby, both Fluoro-Gold and True Blue could impair motor nerve function. The tibial functional index measured at 3 and 14 days after Fluoro-Gold or True Blue injection indicated that motor nerve fibers underwent degeneration rather than temporary loss of conduction capability, i.e. neurapraxia. This observation was confirmed by significant atrophy in corresponding muscles and histological examinations of the nerve. Additionally, True Blue caused less severe impairment of motor nerve function than Fluoro-Gold.
One interesting finding of this study was that intra-fascicular injection of Fluoro-Gold solution into the tibial nerve resulted in significant atrophy, with varying degrees, of the tibialis cranialis muscle which is innervated by the peroneal nerve. One possible explanation was that the peroneal nerve was contaminated by the tracer. Whatever the mechanism is, the present study indicated that Fluoro-Gold could obviously lead to greater damage to the nerve, demonstrating its potent neuronal tracer activity[13]. This observation might explain why only the Fluoro-Gold group exhibited sensory impairment in the middle plantar skin, an area co-innervated by a branch of the peroneal nerve which might be exposed to Fluoro-Gold due to dye leakage[24]. Although Fluoro-Gold is claimed to be non-toxic[25,26], many studies have demonstrated the contrary, showing that Fluoro-Gold is capable of being transported through nerve fibers and exhibiting significant retrograde labeling, and results in neural degeneration and neuronal death[14,27,28].
The present study indicated that Fluoro-Gold caused local necrosis, which is in accordance with previous findings[13].
Previous studies have demonstrated that neurogenic vasodilatation, a phenomenon resulting from stimulation of afferent C fibers and release of afferent neurotransmitters like calcitonin gene-related peptides, can be induced by nerve injury[20,29].
In this study, the irritation of tibial afferent C fibers was evaluated by laser Doppler perfusion imaging. Results showed that injection of Fluoro-Gold or True Blue significantly led to neurogenic vasodilatation, in accordance with tibial nerve transection injury but in contrast to Fluoro-Ruby or saline injection. Schmelz et al[30] demonstrated that axonal reflex flare or neurogenic vasodilatation in human skin is mediated by mechano-insensitive C-nociceptors. This indicated that Fluoro-Gold and True Blue were both toxic to afferent C fibers, at least mechano-insensitive C-nociceptors.
To identify whether the procedure of intra-neural injection affected nerve function, a control group of saline injection was included in this study, and the results indicated that injection of saline into a nerve did not affect nerve function. This is similar to a previous finding shown via electrophysiological examinations by Puigdellivol-Sanchez et al[26].
Fluoro-Gold, True Blue and Fluoro-Ruby are all recognized as retrograde neuronal tracers, labeling neuronal perikaryon via axoplasmic transport[13,14,26]. Although tracers are typically applied with a conduit reservoir approach, the injection method is often utilized not only because it is more convenient and less time-consuming but because it is usually the only way to deliver tracers to the central nervous system[6,28,31].
The results of this study showed that labeling efficacy of tracers seemed to correlate with neural toxicity. Fluoro-Gold is a potent tracer with higher labeling efficacy than True Blue and Fluoro-Ruby, because of significantly more chemical severance of nerve fibers and thus easier uptake by axons, as evidenced by histological examination. By contrast, Fluoro-Ruby exhibited no apparent toxicity to an intact nerve, but gave far less effective retrograde labeling, possibly because of poor permeability through the endoneurium and Schwann cell basal lamina.
Previous studies also indicate that it is practicable for Fluoro-Ruby use to cut the nerve and immerse the proximal nerve stump into tracer solution[13,32]. Although True Blue was found to cause neural degeneration and functional impairment in this study, no labeled neuron loss was observed after long-term labeling by True Blue[14]. This evidence indicated that the neural toxicity elicited by True Blue might be temporary, but further investigation is still required.
In conclusion, the present study indicated that exposure of an intact nerve to Fluoro-Gold or True Blue might hinder neurological function and Fluoro-Gold seemed more toxic, while Fluoro-Ruby caused no functional impairment of the nerve with pressure injection-based tracing. To the best of our knowledge, this is the first known study addressing the issue of tracer influence on neurological function in the peripheral nervous system, suggesting that functional deficit may also result from tracing techniques used in identifying neuronal projections in the central nervous system. This observation may need to be taken into account in relevant studies. This study provided new insight into tracer toxicity with respect to neurological function, suggesting that the first fluorescent tracer should be cautiously selected when sequential retrograde tracing is utilized to evaluate the accuracy of neural regeneration.
Further investigations are indicated to determine the underlying mechanisms of neurotoxicity seen with potent tracers, and to address the possibility of restructuring the tracer molecule so that the efficacy of a potent tracer remains with minimized toxicity.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
This study was performed in Jiangsu Key Laboratory of Neuroregeneration, Nantong University, China from March 2012 to August 2012.
Materials
Animals
Sixty adult female Sprague-Dawley rats (production license No. SCXK (Su) 2008-0010; application license No. SYXK (Su) 2007-0021), weighing 220–250 g, were used in this study.
Methods
Tracing surgery
Rats were anesthetized with intraperitoneal injection of 3% sodium pentobarbital solution (30 mg/kg body weight) and the left lateral thigh was prepared for surgery. The left tibial nerve was exposed and injected with 5 μL of tracer solution/suspension, using a 10-μL microsyringe. For Fluoro-Gold, True Blue and Fluoro-Ruby groups, the left tibial nerve was injected with 5% Fluoro-Gold (Fluorochrome LLC, Denver, CO, USA) solution, 4% True Blue (Invitrogen, Carlsbad, CA, USA) suspension and 10% Fluoro-Ruby (Fluorochrome, LLC) solution, respectively. The tracers were dissolved or suspended in sterile saline, and the needle of the microsyringe was allowed to remain in position for 10 minutes to avoid tracer leakage. The concentration and delivery of tracers were chosen according to previous literature[6,33,34,35,36]. Sterile saline (5 μL) was injected into the saline group, and the exposed tibial nerve was left intact in the sham surgery group.
The tibial nerve transection group was used as a denervation control, in which the tibial nerve was transected at 5 mm distal to the sciatic nerve, with the proximal nerve stump being sutured to the adjacent muscle. Incisions were properly closed and the rats were housed and fed ad libitum under a 12-hour light/dark cycle.
Laser Doppler perfusion imaging analysis
Neurogenic vasodilatation tests were performed to assess the effect of tracer exposure on afferent C fibers[30]. Immediately after surgery, all rats were subjected to laser Doppler perfusion imaging analysis[37] of both hind paws under anesthesia, using the PeriScan PIM 3 system (Perimed AB, Järfälla, Sweden). Briefly, rats were placed in a prone position, leaving the plantar side of both hind paws exposed to the laser beam. The hind paws were scanned twice with the repeated scan mode at 55 × 14 low resolution. The mean percentage of blood perfusion volume on the operated side compared with the contralateral normal side was calculated using the LDPIwin software package (Perimed AB, Järfälla, Sweden)[20].
Plantar sensation test
The possible impact of tracer exposure on sensory nerve function was evaluated with the plantar test at 5 days after tracer injection, using an infrared heat-based plantar test instrument (UGO Basile, Comerio, Italy). Briefly, rats were habituated in a transparent plastic cage for over 30 minutes, and the middle plantar surface of the left foot was exposed to an infrared beam. The onset latency of foot withdrawal and licking was automatically recorded. The cut-off value was set as 20.1 seconds to avoid burn injury. Each rat was tested three times with 1-hour intervals, and the mean latency was calculated.
Walking track analysis
Rats were subjected to walking track analysis to evaluate the effect of tracer injection on motor nerve function at 3 and 14 days postoperatively.
Briefly, the plantar sides of both hind feet were painted with non-toxic red ink, and rats were allowed to walk and pass a 42.0 cm × 8.2 cm track, leaving foot prints on the paper. The basic parameters, namely print length (PL), toe spread (TS) and intermediate toe spread (IT), were measured from both experimental (E) and contralateral normal (N) sides as described previously[38,39].
Tibial functional index was calculated as follows: TFI = −37.2 × [(EPL–NPL)/NPL] + 104.4 × [(ETS–NTS)/NTS] + 45.6 × [(EIT–NIT)/NIT]−8.8. The value of −100 represents complete loss of tibial nerve function, and 0 for normal function.
Muscle weight ratio
At 14 days after tracer injection, all rats were deeply anesthetized and sacrificed by transcardial perfusion of saline, followed by 1.25% glutaraldehyde plus 1% paraformaldehyde in 0.1 mol/L phosphate buffer. The gastrocnemius, soleus and tibialis cranialis muscles were carefully dissected out and weighed, and the wet weight ratio of muscles was calculated by dividing muscle weight of the operated side by that of the contralateral normal side[6,20].
Histological examination
The tibial nerve just distal to the injection site was dissected out and fixed by immersion in 4% glutaraldehyde at 4°C overnight, post-fixed with 1% osmium tetraoxide, dehydrated in ethanol, and embedded in Epon 812 epoxy resin. Semi-thin transverse sections of the nerve specimens were prepared and stained with toluidine blue for microscopy.
The lumbar enlargement of the spinal cord, ranging from L2 to S1, in five rats randomly selected from each of Fluoro-Gold, True Blue and Fluoro-Ruby groups, was dissected out and postfixed in 4% buffered paraformaldehyde at 4°C overnight. The following morning tissue was sequentially dehydrated in 10, 20 and 30% sucrose in 0.1 mol/L phosphate buffer. The spinal cord specimen was longitudinally and serially frozen-sectioned at a thickness of 30 μm on a cryostat.
Sections were then mounted on 10% poly-L-lysine pre-coated slides, and labeled motor neurons with visible nuclei were counted in every second slice under a DMR fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Data were analyzed with GraphPad Prism 4 software package (GraphPad Software Inc, CA, USA). For quantitative data of laser Doppler perfusion imaging analysis, non-parametrical Kruskal-Wallis test, in combination with Dunn's Multiple Comparison Test, was utilized. For the remaining quantitative data, one-way analysis of variance with Student-Newman-Keuls post hoc test was used. A P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was financially supported by the National High-Tech Research and Development Program of China (863 Program), No. 2012AA020502; the National Natural Science Foundation of China, No. 81100939 and 81130080; the Collegiate Natural Science Foundation of Jiangsu Province, No. 10KJB310009; the Innovation Program for Collegiate Postgraduates of Jiangsu Province, No. CXZZ12_0872; and the Qinglan Project of Jiangsu Province.
Conflicts of interest: None declared.
Ethical approval: All animal experiments were approved by the Ethics Committee for Laboratory Animals at Nantong University in China.
(Reviewed by Aprico K, Norman C, Bian LG, Huang F)
(Edited by Wang J, Yang Y, Li CH, Song LP)
REFERENCES
- 1.Wang Q, Sporns O, Burkhalter A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J Neurosci. 2012;32(13):4386–4399. doi: 10.1523/JNEUROSCI.6063-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beier KT, Saunders A, Oldenburg IA, et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc Natl Acad Sci U S A. 2011;108(37):15414–15419. doi: 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Gao E, Burkhalter A. Gateways of ventral and dorsal streams in mouse visual cortex. J Neurosci. 2011;31(5):1905–1918. doi: 10.1523/JNEUROSCI.3488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennington JB, Pope S, Goodheart AE, et al. Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J Neurosci. 2011;31(37):13078–13087. doi: 10.1523/JNEUROSCI.1197-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Wang HL, Li X, et al. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31(23):8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Ding F, Wu J, et al. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials. 2007;28(36):5526–5535. doi: 10.1016/j.biomaterials.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson SC, Tester NJ, Howland DR. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J Neurosci. 2011;31(15):5710–5720. doi: 10.1523/JNEUROSCI.4459-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Hu W, Cao Y, et al. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128(Pt 8):1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- 9.da Silva LF, Coimbra NC, Menescal-de-Oliveira L. Rostral ventromedial medulla connections in Cavia porcellus and their relation with tonic immobility defensive behavior: a biotinylated dextran amine neurotracing study. Neurosci Lett. 2013;535:116–121. doi: 10.1016/j.neulet.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Nordblom J, Persson JK, Aberg J, et al. FGF1 containing biodegradable device with peripheral nerve grafts induces corticospinal tract regeneration and motor evoked potentials after spinal cord resection. Restor Neurol Neurosci. 2012;30(2):91–102. doi: 10.3233/RNN-2011-0623. [DOI] [PubMed] [Google Scholar]
- 11.de Ruiter GC, Spinner RJ, Malessy MJ, et al. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63(1):144–153. doi: 10.1227/01.NEU.0000335081.47352.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobbert C, Apps R, Bechmann I, et al. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62(4):327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi A, Moradzadeh A, Hunter DA, et al. Retrograde labeling in peripheral nerve research: it is not all black and white. J Reconstr Microsurg. 2007;23(7):381–389. doi: 10.1055/s-2007-992344. [DOI] [PubMed] [Google Scholar]
- 14.Garrett WT, McBride RL, Williams JK, Jr, et al. Fluoro-Gold's toxicity makes it inferior to True Blue for long-term studies of dorsal root ganglion neurons and motoneurons. Neurosci Lett. 1991;128(1):137–139. doi: 10.1016/0304-3940(91)90778-r. [DOI] [PubMed] [Google Scholar]
- 15.Franz CK, Rutishauser U, Rafuse VF. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 2008;131(Pt 6):1492–1505. doi: 10.1093/brain/awn039. [DOI] [PubMed] [Google Scholar]
- 16.Hu N, Wu H, Xue C, et al. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34(1):100–111. doi: 10.1016/j.biomaterials.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Choi D, Li D, Raisman G. Fluorescent retrograde neuronal tracers that label the rat facial nucleus: a comparison of Fast Blue, Fluoro-ruby, Fluoro-emerald, Fluoro-Gold and DiI. J Neurosci Methods. 2002;117(2):167–172. doi: 10.1016/s0165-0270(02)00098-5. [DOI] [PubMed] [Google Scholar]
- 18.Katada A, Vos JD, Swelstad BB, et al. A sequential double labeling technique for studying changes in motoneuronal projections to muscle following nerve injury and reinnervation. J Neurosci Methods. 2006;155(1):20–27. doi: 10.1016/j.jneumeth.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Tomita K, Kubo T, Matsuda K, et al. Effect of conduit repair on aberrant motor axon growth within the nerve graft in rats. Microsurgery. 2007;27(5):500–509. doi: 10.1002/micr.20394. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Yang M, Chang J, et al. Laser doppler perfusion imaging of skin territory to reflect autonomic functional recovery following sciatic nerve autografting repair in rats. Microsurgery. 2012;32(2):136–143. doi: 10.1002/micr.20974. [DOI] [PubMed] [Google Scholar]
- 21.Jungnickel J, Haastert K, Grzybek M, et al. Mice lacking basic fibroblast growth factor showed faster sensory recovery. Exp Neurol. 2010;223(1):166–172. doi: 10.1016/j.expneurol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Lewin SL, Utley DS, Cheng ET, et al. Simultaneous treatment with BDNF and CNTF after peripheral nerve transection and repair enhances rate of functional recovery compared with BDNF treatment alone. Laryngoscope. 1997;107(7):992–999. doi: 10.1097/00005537-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Yuan X, Ding F, et al. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17(17-18):2231–2244. doi: 10.1089/ten.TEA.2010.0633. [DOI] [PubMed] [Google Scholar]
- 24.Povlsen B, Stankovic N, Danielsson P, et al. Fiber composition of the lateral plantar and superficial peroneal nerves in the rat foot. Anat Embryol (Berl) 1994;189(5):393–399. doi: 10.1007/BF00185434. [DOI] [PubMed] [Google Scholar]
- 25.Novikova L, Novikov L, Kellerth JO. Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods. 1997;74(1):9–15. doi: 10.1016/s0165-0270(97)02227-9. [DOI] [PubMed] [Google Scholar]
- 26.Puigdellivol-Sanchez A, Valero-Cabre A, Prats-Galino A, et al. On the use of fast blue, fluoro-gold and diamidino yellow for retrograde tracing after peripheral nerve injury: uptake, fading, dye interactions, and toxicity. J Neurosci Methods. 2002;115(2):115–127. doi: 10.1016/s0165-0270(01)00532-5. [DOI] [PubMed] [Google Scholar]
- 27.Dado RJ, Burstein R, Cliffer KD, et al. Evidence that Fluoro-Gold can be transported avidly through fibers of passage. Brain Res. 1990;533(2):329–333. doi: 10.1016/0006-8993(90)91358-n. [DOI] [PubMed] [Google Scholar]
- 28.Schmued LC, Beltramino C, Slikker W., Jr Intracranial injection of Fluoro-Gold results in the degeneration of local but not retrogradely labeled neurons. Brain Res. 1993;626(1-2):71–77. doi: 10.1016/0006-8993(93)90564-4. [DOI] [PubMed] [Google Scholar]
- 29.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30(1):5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 30.Schmelz M, Michael K, Weidner C, et al. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport. 2000;11(3):645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- 31.Evans GR, Brandt K, Niederbichler AD, et al. Clinical long-term in vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. J Biomater Sci Polym Ed. 2000;11(8):869–878. doi: 10.1163/156856200744066. [DOI] [PubMed] [Google Scholar]
- 32.Haninec P, Kaiser R, Bobek V, et al. Enhancement of musculocutaneous nerve reinnervation after vascular endothelial growth factor (VEGF) gene therapy. BMC Neurosci. 2012;13:57. doi: 10.1186/1471-2202-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine EG, Decosterd I, Papaloizos M, et al. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15(4):589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 34.Zele T, Sketelj J, Bajrovic FF. Efficacy of fluorescent tracers in retrograde labeling of cutaneous afferent neurons in the rat. J Neurosci Methods. 2010;191(2):208–214. doi: 10.1016/j.jneumeth.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Puder BA, Papka RE. Hypothalamic paraventricular axons projecting to the female rat lumbosacral spinal cord contain oxytocin immunoreactivity. J Neurosci Res. 2001;64(1):53–60. doi: 10.1002/jnr.1053. [DOI] [PubMed] [Google Scholar]
- 36.Coomes DL, Schofield RM, Schofield BR. Unilateral and bilateral projections from cortical cells to the inferior colliculus in guinea pigs. Brain Res. 2005;1042(1):62–72. doi: 10.1016/j.brainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Bonelli RM, Koltringer P. Autonomic nervous function assessment using thermal reactivity of microcirculation. Clin Neurophysiol. 2000;111(10):1880–1888. doi: 10.1016/s1388-2457(00)00424-7. [DOI] [PubMed] [Google Scholar]
- 38.Varejao AS, Meek MF, Ferreira AJ, et al. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J Neurosci Methods. 2001;108(1):1–9. doi: 10.1016/s0165-0270(01)00378-8. [DOI] [PubMed] [Google Scholar]
- 39.Fey A, Schachner M, Irintchev A. A novel motion analysis approach reveals late recovery in C57BL/6 mice and deficits in NCAM-deficient mice after sciatic nerve crush. J Neurotrauma. 2010;27(5):815–828. doi: 10.1089/neu.2009.1217. [DOI] [PubMed] [Google Scholar]


