Abstract
Objectives/Hypothesis
Cigarette smoke exposure is a significant risk factor in the development of otitis media. NF-κB is a transcription factor known to mediate cigarette smoke effects in multiple cell types. We hypothesized that stimulation of murine middle ear epithelial cells (MEEC) with cigarette smoke condensate (CSC) activates NF-κB resulting in upregulation of proinflammatory cytokines.
Study Design
In vitro model of cultured murine middle ear epithelial cells.
Methods
Time course CSC stimulation of MEEC was performed. Antibody microarrays were then utilized to simultaneously measure 40 inflammatory cytokines. Enzyme-linked immunosorbent assay (ELISA) and quantitative reverse transcriptase-polymerase chain reaction were performed to validate and further evaluate array results. Luciferase reporter assays were performed to evaluate NF-κB activation with CSC in MEEC. Chromatin immunoprecipitation (ChIP) assays were performed to determine whether CSC induces NF-κB interaction with the Tnf-α promoter.
Results
Multiple cytokines showed significant increases with CSC exposure. ELISA studies demonstrated that Tnf-α secretion increased the most. CSC stimulation likewise increased Tnf-α mRNA abundance and induced promoter activity 4.8-fold in a Tnf-α reporter plasmid. Reporter assays demonstrated 4.84-fold activation of NF-κB with CSC. ChIP assays demonstrated NF-κB binding to canonical κB sites in the Tnf-α promoter with CSC stimulation.
Conclusions
CSC activates NF-κB in MEEC. Furthermore, this activation results in CSC induced Tnf-α promoter activation, gene expression, and levels in cell secretions.
Keywords: Tnf-α, mouse middle ear epithelial cells, NF-κB, cigarette smoke condensate
INTRODUCTION
Otitis media (OM) is a ubiquitous condition of early childhood accounting for 16 million physician office visits a year1 at a national cost of $3 to $6 billion.2 Exposure to tobacco smoke in children has been shown to be a significant risk factor in the development of OM. Links between environmental tobacco smoke and OM have been promulgated by various federal agency reports: the Surgeon General,3 the National Research Council,4 the US Environmental Protection Agency (EPA),5 and the National Cancer Institute/California EPA.6 Other environmental and genetic risk factors, such as day care and family history of OM, are associated with middle ear disease in childhood.7–10 In the presence of smoke exposure, the combined interaction of factors might mean the difference in becoming otitis-prone or not.11 Despite strong casual epidemiologic data, direct mechanisms by which cigarette smoke exposure contributes to middle ear pathology are significantly underinvestigated, although inflammation appears to play a significant role.
Proinflammatory cytokines present in middle ear effusion correlate with the degree of middle ear pathology and are actively involved in the inflammatory progression acute to chronic OM.12 TNF-α is an immunostimulating cytokine central to many acute inflammatory cascades that has been identified in a vast majority of pediatric middle ear effusions.13–16 In the middle ear, it has been postulated to contribute to chronic OM pathogenesis by stimulating epithelial mucin gene expression.17 Murine Tnf-α genetic expression is induced by NF-κB through direct cis-interaction with canonical κB sites in the Tnf-α promoter.18 Polymorphisms in the TNF-α gene have been associated with increased risk for chronic otitis media susceptibility and tympanostomy tube placement.19
Multiple genes increased in inflammatory disease states are regulated by NF-κB, a ubiquitous DNA-binding transcription factor.20 Under normal conditions NF-κB is present in the cytoplasm bound to IκB-α, rendering it inactive. Upon extracellular stimulation IκB-α is phosphorylated, leading to its dissociation from the NF-κB subunits and subsequent degradation.21,22 NF-κB then becomes activated, translocating into the nucleus and inducing transcription of multiple genes, including IκB-α. In fact, assaying for NF-κB binding to the IκB-α promoter represents a direct means of measuring for NF-κB activity in cells.23 NF-κB is a known mediator of cigarette smoke effects on gene regulation in multiple cell types.24–26 Its activated form has been shown to be increased in bronchial biopsies of smokers.27 In rabbit middle ear epithelial cells NF-κB has been identified as a key intracellular modulator of chronic inflammation.28 Sustained inflammation in the middle ear is critical in the progression of acute to chronic OM.12,29
For this study, we hypothesized that in vitro stimulation of murine middle ear epithelial cells (MEEC) with cigarette smoke condensate (CSC) activates NF-κB, which subsequently induces Tnf-α proinflammatory cytokine gene expression and protein secretion.
MATERIALS AND METHODS
Cell Lines
The mouse middle ear epithelial cell line (mMEEC) was graciously provided by Dr. Jizhen Lin (University of Minnesota, Minneapolis, MN). These cells are immortalized by a temperature sensitive simian virus 40 (SV-40), allowing for a proliferative phenotype at 33°C and for differentiation at 37°C.30 The mMEEC were maintained and passaged in full growth media (FGM) as previously described.31 Prior to experimentation, cells were transferred to a 37°C, 5% CO2 humidified atmosphere to inactivate the SV-40 virus.
Cigarette Product Cell Exposure
CSC was purchased from Murty Pharmaceuticals (Lexington, KY) where it was prepared using a Phipps-Bird 20-channel smoking machine. The particulate matter from Kentucky standard cigarettes (1R3F; University of Kentucky, Lexington, KY) was collected on Cambridge glass fiber filters and the amount obtained determined by weight increase of the filter. CSC was prepared by dissolving the collected smoke particulates in dimethyl sulfoxide (DMSO) to yield a 4% solution (w/v). The average yield of CSC was 26.1 mg/cigarette. The CSC was diluted into DMSO and aliquots were kept at −80°C.
For experiments, middle ear cells were stimulated with 0 to 40 μg/mL of CSC in serum-free FGM (SFM), concentrations known to stimulate cytokine secretion in cultured endothelial cells,32 for the times indicated in the figure legends. Secondhand smoke has been shown to have concentrations estimated to be in the range of this experimental dosing.33
Cytokine Microarray Assays
Cells were grown in T-75 flasks on plastic until they achieved confluency. The mMEEC medium was collected 2, 8, and 24 hours after treatment with CSC or DMSO in SFM. The RayBio Mouse Inflammation Antibody Array (Raybiotech, Norcross, GA) cytokine microarray assay in a sandwich enzyme-linked immunosorbent assay (ELISA) format was used to detect secretion of 40 inflammatory cytokines simultaneously from mMEEC after CSC stimulation. Preblotted membranes with various anticytokine antibodies were incubated with culture medium for 2 hours at room temperature after blocking nonspecific binding sites per the manufacturer’s instructions. After adding a biotin-conjugated anticytokine antibody mixture, the membrane was incubated with HRP-conjugated streptavidin per the manufacturer’s instructions. Sample signal intensity was quantified by densitometry and normalized to the positive control bands from the same membrane to account for exposure and intensity differences from membrane to membrane. Experiments were performed in duplicate three times.
ELISA
Cells were plated onto T-25 flasks at 35,000 cells per flask and grown to confluence. They were then stimulated under the same conditions used for the cytokine microarray experiments. After 0.5, 2, 8, and 24 hours the media was collected, aliquoted, and frozen at −80°C. ELISA for Tnf-α was performed using kits from R&D Systems, Inc. (Minneapolis, MN) as per the manufacturer’s instructions.
Cell Viability Assays
Cell viability was measured by 3-(4,5-dimethylthiazoyl-2-yl) 2,5 diphenyltetrazolium bromide (MTT) assay. Cells were plated onto 96-well plates (5,000 cells/well) and grown at 33°C in a 5% carbon dioxide (CO2)-humidified atmosphere for 48 hours, at which time the medium was changed to SFM at 37°C. The mMEEC were exposed to 2-fold serial dilutions of 2.5 to 80 μg/mL of CSC or DMSO for 2, 8, and 24 hours, 6 wells per condition. The MTT colorimetric assay was performed (Sigma-Aldrich, St. Louis, MO) as per the manufacturer’s instructions.
Preparation of Nuclear Extracts
Cells were grown in T-75 flasks to 60% to 80% confluence and then stimulated with 10, 20, or 40 μg/mL of CSC in DMSO or plain DMSO for 60 minutes. Cell suspensions were prepared by Trypsin-ethylenediaminepentaacetic acid (EDTA) treatment, and cells were washed twice in cold phosphate buffered saline (PBS) and pelleted at 500 × g. Cytoplasmic and nuclear protein extraction was performed using a kit from Pierce (Rockford, IL) as previously described.31 The protein concentrations of the extracts were determined in triplicate by using the bicinchoninic acid modification of the biuret reaction (Pierce Protein Assay Kit; Pierce) scaled for microtiter plate analysis. Bovine serum albumin was used as a standard, and the plates were read at 580 nm on a Biotek 311 microtiter plate reader. Standard curves were generated using computer software. The correlation coefficients for the functions were greater than 0.95 in all experiments.
Construction of Tnf-α Promoter Plasmid
The following primers tagged with 5′ restriction enzyme sequences were obtained from Integrated DNA Technologies (Coralville, IA): F 5′ GACTCTCGAGGGGTGACCAAGGGGT TCTAT 3′ and R 5′ GACTAAGCTTCTGGCTAGTCCCTTG CTGTC 3′ (XhoI restriction sites on the F primers’ 5′ end, and HindIII restriction site on the R primer 5′ end). Polymerase chain reaction (PCR) was performed using these primers and mouse liver genomic DNA as template. After purification, resulting products were restricted and ligated to the PGL3 vector (Promega, Madison, WI). After confirmation of the products by DNA sequencing the −1332 nucleotide base pair TnfαLuc promoter plasmid (TnfαLuc) was transformed in competent DH5α cells and amplified with MaxiPrep kits (Promega).
Transient Transfection and Luciferase Assays
The pIgκBLuc reporter construct containing three immunoglobulin G-κ chain NF-κB binding sites upstream of the luciferase gene has been previously described34 and was generously provided by Frank Ondrey, MD, PhD, University of Minnesota, Minneapolis, Minnesota. This NF-κB reporter plasmid or TnfαLuc was transiently transfected into mMEEC cells for luciferase assays as follows. Cell line cultures at 70% confluence were cotransfected with the luciferase plasmids (2 μg/mL) and a pCMV-βGal reporter construct (0.4 μg/mL) (Clontech, Mountainview, CA) in Opti-MEM medium containing 3 μg/mL of Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 6 hours, the medium was removed, and the cells were placed in FGM. The next day the cells were treated with CSC at 10, 20, and 40 μg/ml in DMSO, or plain DMSO for 8 hours. After stimulation, the relative luciferase activity was determined with the Dual Light reporter gene assay (Tropix, Medford MA) and a Mono-light 2010 plate luminometer (Analytical Luminescence Laboratories, San Diego, CA) according to the manufacturers’ instructions. Results for relative luciferase units (RLU) were determined as a ratio of the luciferase constructs over the pCMV-βGal reporter to normalize for harvesting efficiency.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction Analyses of Tnf-α Gene Expression
RNA was extracted from mMEEC cells exposed to CSC (10, 20, 40 μg/mL) or vehicle for 2, 8, and 24 hours using TRIzol (Invitrogen). RNA was precipitated with isopropyl alcohol, washed, and dissolved in RNase-free water. Reverse transcription reaction was then performed by using 1 μg of total RNA from each sample and the SuperScript III reverse transcriptase enzyme (Invitrogen), as previously described.31 Generated cDNA was used for PCR using specific pairs of primers as follows: Tnf-α forward primer, 5′-CCCTCACACTCAGATCATCT TCT-3′; Tnf-α reverse primer, 5′-GCTACGACGTGGGCTACAG-3′. β-actin was used as an internal control, and primers for mouse β-actin were obtained from GeneLink (Hawthorne, NY). Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on the generated cDNA products in the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA) using the Power SYBR Green PCR Master Mix from Applied Biosystems (Branchburg, NJ). The β-actin was unchanged by CSC exposure and was used as an internal control for normalizing Tnf-α mRNA levels in control and experimental samples. Dilution curves confirmed the linear dependence of the threshold cycles on the concentration of template RNA samples. Relative quantification of Tnf-α mRNA in control and experimental samples was obtained using the standard curve method.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) procedure was performed using a kit purchased from Upstate Biotechnology (Lake Placid, NY), according to the protocol by the manufacturer. Briefly, after CSC stimulation for 1 hour, cells were incubated with formaldehyde at a final concentration of 1% at 37°C for 10 minutes. Cells were washed twice with ice-cold PBS and were collected by centrifugation at 4°C, and resuspended in the cell lysis buffer (50 mM Tris-HCl, pH 8, 10 mM EDTA, 1% sodium dodecyl sulfonate (SDS), and protease inhibitors). Cell lysates were sonicated to give a DNA size range from 300 to 600 bp, and supernatants were diluted with dilution buffer (16.7 mM Tris-HCl, pH 8, 1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, 0.01% SDS, and protease inhibitors). The solutions were pre-cleared with salmon sperm DNA/protein G agarose slurry and then treated with 10 μg p65 antibody (Rockland, Gilbertsville, PA) overnight at 4°C. Immune complexes were collected by adding a salmon sperm DNA/protein G agarose slurry. The beads were washed sequentially in the following buffers: low salt wash buffer (20 mM Tris-HCl, pH 8.1, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, and 1% Triton X-100); high salt wash buffer (20 mM Tris-HCl, pH 8.1, 500 mM NaCl, 2 mM EDTA, 0.1% SDS, and 1% Triton X-100); LiCl wash buffer (10 mM Tris-HCl, pH 8.1, 0.25 M LiCl, 1% Nonidet P-40, 1% deoxycholate, and 1 mM EDTA), and Tris-EDTA buffer. Immuno-complexes were extracted from the beads with 1% SDS and 0.1 M NaHCO3. Cross-linking was reversed by heating the eluates at 65°C for 4 hours. The eluants were then digested with proteinase K at 45°C for 1 hour, and then further subjected to the phenol/chloroform extraction. The DNA was purified by ethanol precipitation and used as the template in PCR reactions. The region of the Tnf-α promoter containing a proximal NF-κB canonical binding site corresponding to bp −210 and the region of the IκB-α promoter containing a proximal NF-κB canonical binding site corresponding to bp −186 (translation start sites designated as +1) were amplified by PCR. The pair of primers used included the forward primer: 5′-GGGAAACCCCAGGGAAAGAA -3′ and the reverse primer: 5′-TATAAACGCTGGCAGGGGAT -3′ for the Tnf-α promoter and the forward primer: 5′-ACACACACACACCCTCCTGA -3′ and the reverse primer: 5′-TCTCGGTTTCTTCTCCATCG-3′ for the IκB-α promoter. PCR reactions were carried out in 50 μl solutions including 45 μL of PCR Supermix (Invitrogen), 2 μl of input or immunoprecipitated (IP) DNA, and 1.5 μl each of 10 μM forward and reverse primers. PCR products were separated by electrophoresis on a 2% in 1X TAE agarose gel containing 50 ng/mL ethidium bromide and were visualized with a Gel Doc 2000 gel imaging documentation station (Bio-Rad, Hercules, CA). Quantitative RT-PCR was also performed on the input or IP DNA as described above.
STATISTICAL ANALYSIS
The statistical difference between experimental and control groups for all experiments was determined by 2-tailed Student t tests. Significance level was set at P <.05.
RESULTS
CSC Increased Tnf-α Levels in Cultured mMEECsupernatants
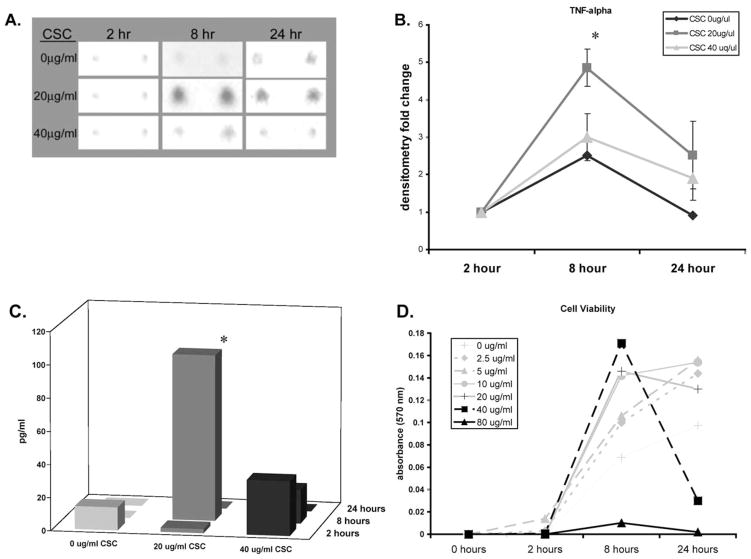
Cytokine microarray analyses demonstrated statistically significant increases in the levels of multiple cytokines in mMEEC cell secretions after 8 hours of exposure to 20 μg/mL CSC (Table I). Notably, of the proinflammatory cytokines previously identified in human adult middle ear effusions such as IL-1β, IL-2, TNF-α, IL-6, and IL-8,16,35–37 Tnf-α was the most increased in these experiments. Figure 1A demonstrates representative results for Tnf-α protein levels analyzed by cytokine microarray blots for one experiment. Three separate experiments were performed. Figure 1B shows the average fold densitometry increases over time for 0, 20, and 40 μg/mL CSC. There was a statistically significant 4.9-fold increase in Tnf-α band intensity after 8-hour stimulation with 20 μg/mL CSC (P = .001). These results were then confirmed by performing ELISA for Tnf-α secretion from cells grown in T-25 flasks and stimulated in separate experiments, but under the same conditions as those performed for the cytokine microarray experiments. At 8 hours after stimulation with 20 μg/mL CSC there was a statistically significant quantitative increase in secreted levels of Tnf-α to 100.47 pg/mL (P = .02) (Fig. 1C). Taken together, these results demonstrated that there were significant increases in TNF-α levels secreted by mMEEC 8 hours after CSC exposure.
TABLE I.
Inflammatory Cytokine Responses to CSC in mMEEC.
| Sig. Increased | No Change | |||
|---|---|---|---|---|
| TNF alpha | IL-1 alpha | IL-9 | Eotaxin-2 | MCP-1 |
| IL-12p40p70 | IL-1 beta | IL-10 | BLC | MCSF |
| MIP-1 alpha | IL-2 | IL-13 | CD30L | Rantes |
| MIP-1 gamma | IL-3 | IL-17 | Fas Ligand | IL-3 |
| MIG | IL-4 | GM-CSF | Fractalkine | SDF-1 |
| TIMP-1 | IL-6 | Eotaxin | IL-8 | |
Cytokine microarray analyses demonstrated statistically significant increases in the levels of multiple cytokines in mMEEC cell secretions after 8 hours of exposure to 20 μg/mL CSC.
CSC = cigarette smoke condensate; mMEEC = mouse murine middle ear epithelial cells.
Fig. 1.
Cigarette smoke condensate (CSC) increases Tnf-α secretion from mouse middle ear epithelial cell line (mMEEC). (A) Detection of Tnf-α in mMEEC culture supernatants after CSC stimulation at the indicated times and doses. Membranes were incubated with secretions, and blots were analyzed by cytokine microarray analyses, as described in the Materials and Methods section. Of the proinflammatory cytokines previously identified in human middle ear effusions,16,42–44 such as IL-1β, IL-2, TNF-α, IL-6, and IL-8; Tnf-α was the only one increased in mMEEC in these studies. (B) Densitometry of three independent experiments of Tnf-α levels showed a 4.9-fold statistically significant increase following exposure to 20 μg/mL of CSC for 8 hours (P = .001). (C) Enzyme-linked immunosorbent assay done on separate experiments showed a statistically significant increase in Tnf-α levels to 100.47 pg/mL following exposure to 20 μg/mL of CSC for 8 hours (P =.02). (D) The 3-(4,5-dimethylthiazoyl-2-yl) 2,5 diphenyltetrazolium bromide cell viability assays demonstrated that exposure to 40 μg/mL CSC for 24 hours resulted in statistically significant decreases in mMEEC cell viability. Exposure to 80 μg/mL resulted in decreased cell viability at all time points studied. The y-axis represents colometric absorbance at 570 nm, a direct measure of viable cells.
Initial increases in secreted Tnf-α noted with 40 μg/mL CSC at 8 hours decreased consistently over time, suggesting prolonged exposure to this dose of CSC is harmful to the cells. For this reason, we performed MTT cell viability assays to determine whether higher doses of CSC were toxic to these cells. Exposure of cells to 40 μg/mL of CSC resulted in significant decreases in mMEEC cell viability after 24 hours. Exposure to 80 μg/mL of CSC resulted in decreased cell viability at all time points studied (Fig. 1D).
CSC Increased Expression of Tnf-μ mRNA in mMEEC
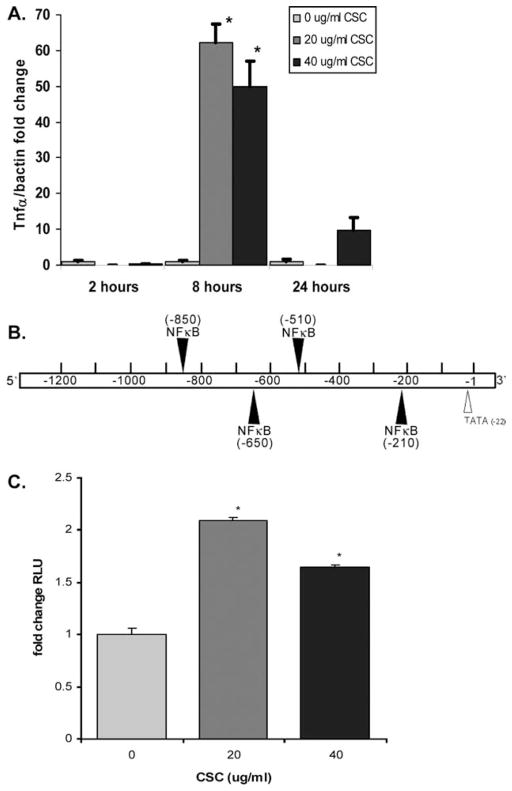
Quantitative real-time RT-PCR results showed statistically significant increases in Tnf-α transcription with CSC (Fig. 2A) at 20 μg/mL (62.4-fold, P = .006) and 40 μg/mL (49.8-fold, P = .02) for 8 hours. At longer exposure times (24 hours) a decrease in upregulation was noted. This is likely due to the decreased cell viability noted in Figure 1D and in other reports38 with higher CSC doses and times. To determine whether the observed increases in Tnf-α mRNA levels with CSC were due to increased promoter activation and not increased message stability, studies were performed to determine whether CSC increased activity of a Tnf-α promoter luciferase construct containing −1332 bp of the Tnf-α 5′ flanking region (Fig. 2B). Luciferase reporter gene assay (Fig. 2C) demonstrated statistically significant relative luciferase unit (RLU) of 2.1, and 1.6-fold activation of the Tnf-α promoter construct in mMEEC with 20 and 40 μg/mL, respectively, of CSC compared to control (P < .001). As shown in Figure 2B, MatInspector analysis version 3.0 (Genomatix MatInspector; www.genomatix.de) shows the presence of four canonical NF-κB cis-sites in the Tnf-α promoter construct.
Fig. 2.
Cigarette smoke condensate (CSC) increases expression of Tnf-α in mouse middle ear epithelial cell line (mMEEC). (A) Real-time reverse transcriptase-polymerase chain reaction analysis showed marked increased expression of Tnf-α mRNA with 20 and 40 μg/mL CSC at 8 hours. (B) Diagram of the proximal −1332 bp of the Tnf-α 5′ flanking region with putative NF-κB sites depicted. This region was cloned onto a PGL3 luciferase vector for reporter assay experiments. (C) Luciferase reporter assays with TnfαLuc demonstrated statistically significant increases in Tnf-α promoter activity with CSC. The results are normalized to β-Gal cotransfection to account for cell number and transfection efficiency.
CSC Increased NF-κB Activity in Middle Ear Epithelial Cells
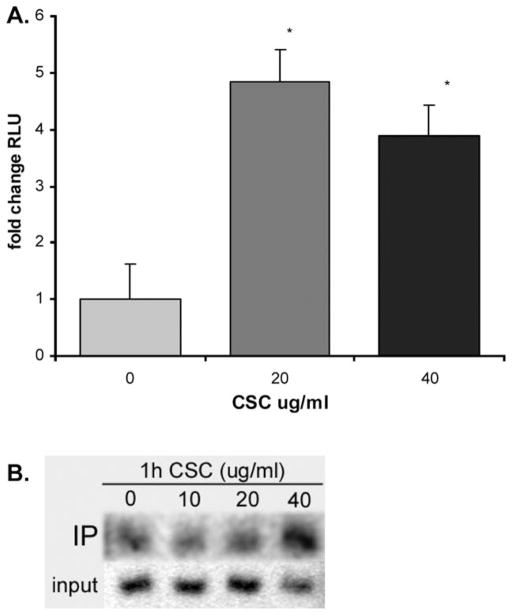
Genetic expression of TNF-α is induced by NF-κB through direct cis-interaction with κB sites in the Tnf-α promoter,18 and NF-κB is a mediator of cigarette smoke effects in multiple epithelial cell types.24–26 To elucidate whether this is also the case in middle ear epithelium, we performed reporter assays in our cells. In mMEEC, luciferase assays with the NF-κB reporter pIgκBLuc demonstrated statistically significant 4.84- and 3.88-fold induction after stimulation with 20 and 40 μg/mL of CSC, respectively, compared to control (Fig. 3A). ChIP assays demonstrated binding of p65 NF-κB subunits to a proximal canonical site in the IκBα promoter after 1 hour of CSC stimulation (Fig. 3C), a definite measure of NF-κB activation, as one of the most potent promoters induced by NF-κB is that of its inhibitor, IκBα. Taken together, the results shown in Figure 3 qualitatively and quantitatively demonstrated that CSC induces NF-κB nuclear translocation and functional activation in middle ear epithelial cells.
Fig. 3.
Cigarette smoke condensate (CSC) increased NF-κB activity in middle ear epithelial cells. (A) The NF-κB reporter plasmid pIgκBLuc containing three consensus κB sites upstream of a luciferase vector demonstrate statistically significant increases in NF-κB activity with 20 and 40 μg/mL CSC in mouse middle ear epithelial cell line (mMEEC). ChIP assays done in mMEEC demonstrated binding of the p65 NF-κB subunit to a proximal canonical site in the IκBα promoter after 1 hour of CSC stimulation.
CSC Induced NFκB Binding to the Tnf-α Promoter
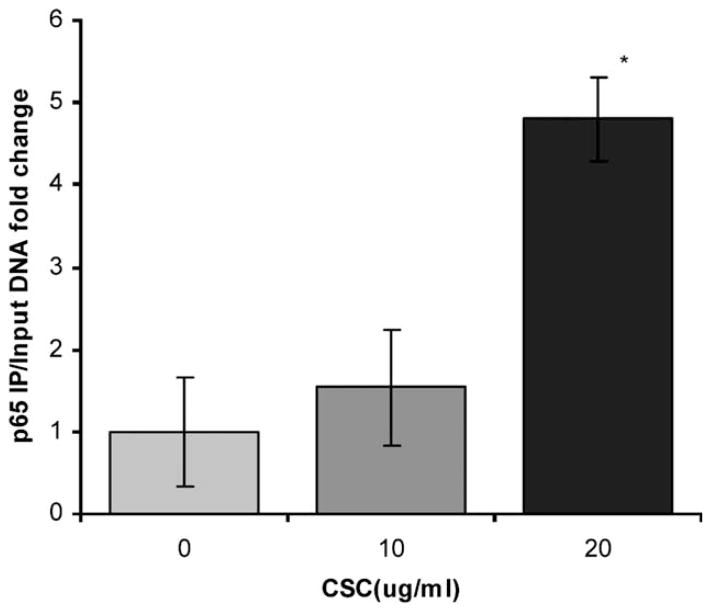
ChIP assays were performed to determine whether NF-κB bound to the Tnf-α promoter following exposure to CSC. Quantitative real-time PCR using primers corresponding to the −216 to −194 sequence on the Tnf-α promoter was carried out with p65 IP cellular DNA template. The data showed 4.7-fold increased levels after 1 hour of exposure to 20 μg/mL CSC compared to control (Fig. 4A). Input (unprecipated, genomic) DNA did not change significantly with CSC stimulation. The data demonstrate that CSC induced NF-κB binding to a consensus kB site on the proximal Tnf-α promoter. Separate experiments underway have shown that NF-κB inhibition mitigates CSC induced Tnf-α transcription in mMEEC (data not shown).
Fig. 4.
Cigarette smoke condensate (CSC) induced NFκB binding to the Tnf-α promoter. Chromatin immunoprecipitation assays demonstrated binding of p65 NF-κB subunit to the kB site in the proximal region of the Tnf-α promoter after 1 hour exposure to 20 μg/mL CSC compared to control.
DISCUSSION
Tobacco smoke exposure has been shown to be a significant epidemiological risk factor for the development of OM,3,4,6,11,39 yet molecular mechanisms that link tobacco smoke exposure to OM are mostly unknown to date. Investigations in bronchial epithelial cells have demonstrated that CSC activates transcription factors, such as NF-κB, that are involved in classic proinflammatory pathways.26 Multiple proinflammatory cytokines have been detected in the middle ear effusions of children with chronic OM undergoing tympanostomy tube insertion.16,35–37 Levels of cytokines in middle ear effusion predicted the time to OM recurrence.15 By comparing levels of cytokines with different stages of middle ear inflammation, some have speculated that cytokines play a pivotal role in the progression of OM from acute to chronic.40 Proposed mechanisms through which proinflammatory cytokines contribute to chronic OM include cellular upregulation of mucins. We recently demonstrated that CSC activates NF-κB and mucin glycoprotein Muc5b gene expression in differentiated mMEEC.31 Others have demonstrated upregulation of the Muc5ac gene in vitro in chinchilla middle ear cells stimulated with TNF-α.41
We hypothesized that CSC activates mouse middle ear epithelial cell line secretion of proinflammatory cytokines. The hypothesis was tested by exposing mMEEC to CSC for defined times and monitoring 40 secreted cytokines simultaneously using a cytokine microarray assay. With the CSC dose (20 μg/mL) previously shown to stimulate maximal Muc5b expression,31 increased secretion of Tnf-α was observed at 8 hours. This was confirmed with ELISA analysis. Notably, of the proinflammatory cytokines previously identified in human middle ear effusions,16,42–44 such as IL-1β, IL-2, TNF-α, IL-6, and IL-8, Tnf-α was the only one increased in mMEEC in these studies. As others have previously demonstrated,38 we also noted that high doses of CSC are toxic to cells in culture. How this exactly translates to the clinical condition of cigarette smoke exposure to OM risk is unclear, but suggests tobacco smoke might be noxious to middle ear epithelium on a transient second hand exposure. More importantly, the MTT data provides a reference for cell viability events expected to occur with in vitro CSC exposure in experiments along the lines of those performed herein. Dosing for future experiments using CSC as an in vitro agent should take into consideration these cell viability effects as well.
To determine whether the increased secretion of Tnf-α was due to increased gene expression, we performed RT-PCR for TNF-α mRNA levels and luciferase reporter analysis for Tnf-α promoter activation. Results demonstrated that indeed CSC stimulation causes activation of the Tnf-α promoter and increased gene expression. These data were not only in line with the secreted protein findings presented in Figure 1, but indicate that tobacco smoke products have the capability of mediating cell effects at the genetic level in cells derived from a middle ear epithelial lineage. In many ways, this finding is not necessarily novel in that this is known to occur in macrophages24 and pulmonary cells.45
The Tnf-α promoter is known to be at least partly under the transcriptional control of NF-κB.18,46,47 Because NF-κB has also been demonstrated to be a major intracellular mediator of cigarette smoke effects in multiple cell types,24–26,38,48 we sought to confirm whether CSC would be able to activate NF-κB in middle ear cell lines derived from mice. Luciferase assays ChIP analysis of a proximal NF-κB site on the IκBα promoter demonstrate that indeed CSC activates NF-κB nuclear translocation and targets kB sites in target genes in cells derived from middle ear epithelium. Notably, the higher dose (40 μg/mL) of CSC induced NF-κB activation by reporter assay and the highest amount of binding to the IκBα promoter in contra-distinction to the Tnf-α secretion and mRNA expression data that showed maximal activation at 20 μg/mL. This is probably explained by the fact that at higher doses or at longer exposure times, CSC affects cell viability and NF-κB beyond its proinflammatory effects, and might also be maximally activated under conditions of threatened cell viability to promote a prosurvival mode (Fig 1D). Measures for Tnf-α secretion and expression were done at 8 hours to allow for occurrence of downstream events related to NF-κB activation. In vivo experiments done in mice demonstrate similar findings in that cigarette smoke exposure results in Tnf-α overexpression through the induction of proinflammatory transcription factors.45
ChIP analysis done with real-time PCR indeed showed that NF-κB binds to the proximal −216 to −194 sequence on the Tnf-α promoter. Site specific mutagenesis directed luciferase constructs are being generated to determine if this and other NF-κB sites are indeed functional. Initial investigations into whether NF-κB inhibition abolishes the CSC induced Tnf-α activation using potent IKK inhibitors is currently being performed in our lab, and initial results that need to be validated indeed point in that direction (data not shown).
CONCLUSION
Dissolved cigarette smoke particulate matter activates the transcription factor NF-κB in middle ear epithelial cells, and this activation induces upregulation of Tnf-α gene expression and results in increased protein in mMEEC secretions. These results indicate that tobacco smoke exposure in the middle ear is able to activate a proinflammatory epithelial phenotype characterized in part by an abundance of Tnf-α release. This molecular event might represent an important mechanism in OM disease progression in children chronically exposed to secondhand cigarette smoke and provides an initial link into how environmental tobacco smoke contributes causally to middle ear disease.
Acknowledgments
This work was supported by a Triological Society Career Development Award and by an Avery Scholar Research Award from Children’s National Medical Center to Diego Preciado, MD.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002 Jun 5;:1–32. [PubMed] [Google Scholar]
- 2.Rosenfeld RM, Casselbrant ML, Hannley MT. Implications of the AHRQ evidence report on acute otitis media. Otolaryngol Head Neck Surg. 2001;125:440–448. doi: 10.1067/mhn.2001.119326. discussion 439. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services. The health consequences of involuntary smoking: a report of the Surgeon General. Washington, DC: US DHHS, Public Health Service, Centers for Disease Control; 1986. DHHS Publication No. [CDC] 87-8398. [Google Scholar]
- 4.National Research Council. Environmental tobacco smoke: measuring exposure and assessing health effects. In: Press NA, editor. Committee on Passive Smoking, Board on Environmental Studies and Toxicology. National Academy Press; Washington, DC: 1986. [Google Scholar]
- 5.US Environmental Protection Agency. Respiratory health effects of passive smoking: lung cancer and other disorders. Washington, DC: USEPA Office of Research and Development; 1992. [Google Scholar]
- 6.National Cancer Institute. Smoking and Tobacco Control Monograph No. 10. US DHHS, National Institutes of Health, National Cancer Institute; Bethesda, MD: 1999. Health effects of exposure to environmental tobacco smoke: the report of the California Environmental Protection Agency. [Google Scholar]
- 7.Daly KA, Giebink GS. Clinical epidemiology of otitis media. Pediatr Infect Dis J. 2000;19:S31–S36. doi: 10.1097/00006454-200005001-00006. [DOI] [PubMed] [Google Scholar]
- 8.Daly KA, Rovers MM, Hoffman HJ, et al. Recent advances in otitis media. 1. Epidemiology, natural history, and risk factors. Ann Otol Rhinol Laryngol Suppl. 2005;194:8–15. doi: 10.1177/00034894051140s104. [DOI] [PubMed] [Google Scholar]
- 9.Dhooge IJ. Risk factors for the development of otitis media. Curr Allergy Asthma Rep. 2003;3:321–325. doi: 10.1007/s11882-003-0092-8. [DOI] [PubMed] [Google Scholar]
- 10.Rovers MM, de Kok IM, Schilder AG. Risk factors for otitis media: an international perspective. Int J Pediatr Otorhinolaryngol. 2006;70:1251–1256. doi: 10.1016/j.ijporl.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Stenstrom C, Ingvarsson L. Otitis-prone children and controls: a study of possible predisposing factors. 2. Physical findings, frequency of illness, allergy, day care and parental smoking. Acta Otolaryngol. 1997;117:696–703. doi: 10.3109/00016489709113462. [DOI] [PubMed] [Google Scholar]
- 12.Wright CG, Meyerhoff WL. Pathology of otitis media. Ann Otol Rhinol Laryngol Suppl. 1994;163:24–26. doi: 10.1177/00034894941030s507. [DOI] [PubMed] [Google Scholar]
- 13.Juhn SK, Garvis WJ, Lees CJ, Le CT, Kim CS. Determining otitis media severity from middle ear fluid analysis. Ann Otol Rhinol Laryngol Suppl. 1994;163:43–45. doi: 10.1177/00034894941030s512. [DOI] [PubMed] [Google Scholar]
- 14.Juhn SK, Tolan CT, Garvis WJ, Cross DS, Giebink GS. The levels of IL-1 beta in human middle ear effusions. Acta Otolaryngol Suppl. 1992;493:37–42. [PubMed] [Google Scholar]
- 15.Skotnicka B, Hassmann E. Cytokines in children with otitis media with effusion. Eur Arch Otorhinolaryngol. 2000;257:323–326. doi: 10.1007/s004059900218. [DOI] [PubMed] [Google Scholar]
- 16.Ondrey FG, Juhn SK, Adams GL. Early-response cytokine expression in adult middle ear effusions. Otolaryngol Head Neck Surg. 1998;119:342–345. doi: 10.1016/S0194-5998(98)70075-0. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Haruta A, Kawano H, et al. Induction of mucin gene expression in middle ear of rats by tumor necrosis factor-alpha: potential cause for mucoid otitis media. J Infect Dis. 2000;182:882–887. doi: 10.1086/315767. [DOI] [PubMed] [Google Scholar]
- 18.Ye J, Wang L, Zhang X, Tantishaiyakul V, Rojanasakul Y. Inhibition of TNF-alpha gene expression and bioactivity by site-specific transcription factor-binding oligonucleotides. Am J Physiol Lung Cell Mol Physiol. 2003;284:L386–L394. doi: 10.1152/ajplung.00134.2002. [DOI] [PubMed] [Google Scholar]
- 19.Patel JA, Nair S, Revai K, et al. Association of proinflammatory cytokine gene polymorphisms with susceptibility to otitis media. Pediatrics. 2006;118:2273–2279. doi: 10.1542/peds.2006-0764. [DOI] [PubMed] [Google Scholar]
- 20.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 21.Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 23.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- 24.Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 25.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 26.Anto RJ, Mukhopadhyay A, Shishodia S, Gairola CG, Aggarwal BB. Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): correlation with induction of cyclooxygenase-2. Carcinogenesis. 2002;23:1511–1518. doi: 10.1093/carcin/23.9.1511. [DOI] [PubMed] [Google Scholar]
- 27.Di Stefano A, Caramori G, Oates T, et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. [DOI] [PubMed] [Google Scholar]
- 28.Barrett TQ, Kristiansen LH, Ovesen T. NF-kappaB in cultivated middle ear epithelium. Int J Pediatr Otorhinolaryngol. 2003;67:895–903. doi: 10.1016/s0165-5876(03)00137-x. [DOI] [PubMed] [Google Scholar]
- 29.Lim DJ, Hermansson A, Hellstrom SO, et al. Recent advances in otitis media. 3. Animal models; anatomy and pathology; pathogenesis; cell biology and genetics. Ann Otol Rhinol Laryngol Suppl. 2005;194:31–41. [PubMed] [Google Scholar]
- 30.Tsuchiya K, Kim Y, Ondrey FG, Lin J. Characterization of a temperature-sensitive mouse middle ear epithelial cell line. Acta Otolaryngol. 2005;125:823–829. doi: 10.1080/00016480510031533. [DOI] [PubMed] [Google Scholar]
- 31.Preciado D, Lin J, Wuertz B, Rose M. Cigarette smoke activates NF kappa B and induces Muc5b expression in mouse middle ear cells. Laryngoscope. 2008;118:464–471. doi: 10.1097/MLG.0b013e3185aedc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordskog BK, Fields WR, Hellmann GM. Kinetic analysis of cytokine response to cigarette smoke condensate by human endothelial and monocytic cells. Toxicology. 2005;212:87–97. doi: 10.1016/j.tox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005;14:396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanno T, Brown K, Siebenlist U. Evidence in support of a role for human T-cell leukemia virus type I Tax in activating NF-kappa B via stimulation of signaling pathways. J Biol Chem. 1995;270:11745–11748. doi: 10.1074/jbc.270.20.11745. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi K, Yagawa M, Ishinaga H, Kishioka C, Harada T, Majima Y. Mucin gene expression in the effusions of otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2003;67:53–58. doi: 10.1016/s0165-5876(02)00361-0. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell KS, Fitzgerald JE, Burleson JA, Leonard G, Carpenter R, Kreutzer DL. Interleukin-8 expression in otitis media. Laryngoscope. 1994;104:989–995. doi: 10.1288/00005537-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi K, Maesako K, Yuta A, Sakakura Y. Interleukin-8 gene expression in middle ear effusions. Ann Otol Rhinol Laryngol. 1994;103:404–407. doi: 10.1177/000348949410300511. [DOI] [PubMed] [Google Scholar]
- 38.Hellermann GR, Nagy SB, Kong X, Lockey RF, Mohapatra SS. Mechanism of cigarette smoke condensate-induced acute inflammatory response in human bronchial epithelial cells. Respir Res. 2002;3:22. doi: 10.1186/rr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilicali OC, Keles N, De er K, Sa un OF, Guldiken Y. Evaluation of the effect of passive smoking on otitis media in children by an objective method: urinary cotinine analysis. Laryngoscope. 2001;111:163–167. doi: 10.1097/00005537-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Smirnova MG, Birchall JP, Pearson JP. Evidence of T-helper cell 2 cytokine regulation of chronic otitis media with effusion. Acta Otolaryngol. 2005;125:1043–1050. doi: 10.1080/00016480510035449. [DOI] [PubMed] [Google Scholar]
- 41.Kerschner JE, Meyer TK, Yang C, Burrows A. Middle ear epithelial mucin production in response to interleukin-6 exposure in vitro. Cytokine. 2004;26:30–36. doi: 10.1016/j.cyto.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Russo E, Smith CW, Friedman EM, Smith EO, Kaplan SL. Cell adhesion molecules and cytokines in middle ear effusions in children with or without recent acute otitis media. Otolaryngol Head Neck Surg. 2004;130:242–248. doi: 10.1016/j.otohns.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Yellon RF, Doyle WJ, Whiteside TL, Diven WF, March AR, Fireman P. Cytokines, immunoglobulins, and bacterial pathogens in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1995;121:865–869. doi: 10.1001/archotol.1995.01890080033006. [DOI] [PubMed] [Google Scholar]
- 44.Yellon RF, Leonard G, Marucha P, et al. Demonstration of interleukin 6 in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1992;118:745–748. doi: 10.1001/archotol.1992.01880070075014. [DOI] [PubMed] [Google Scholar]
- 45.Li YT, He B, Wang YZ. Exposure to cigarette smoke upregulates AP-1 activity and induces TNF-alpha overexpression in mouse lungs. Inhal Toxicol. 2009;21:641–647. doi: 10.1080/08958370802322596. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Sidiropoulos P, Song G, et al. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- 47.Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–4052. [PubMed] [Google Scholar]
- 48.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]