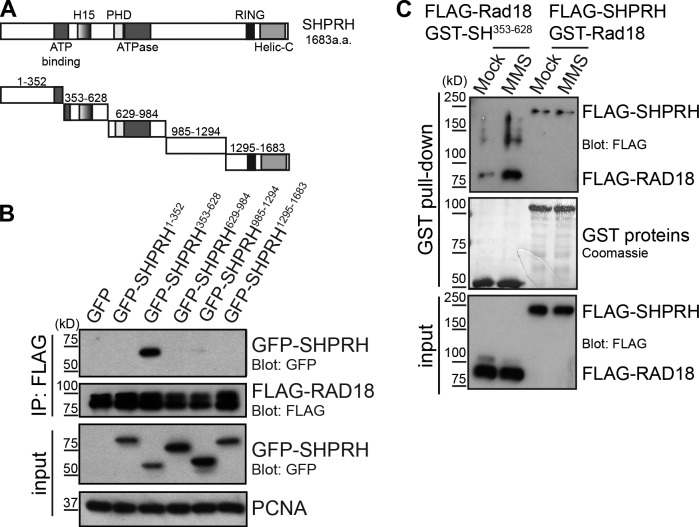
Figure 1.
MMS-induced modification of Rad18 promotes its interaction with SHPRH. (A) Domain structure of SHPRH. Helic-C, helicase C-terminal domain; PHD, plant homeodomain. (B) The SHPRH353–628 (SH353–628) fragment of SHPRH interacts with Rad18. GFP-tagged SHPRH fragments shown in A were expressed in cells with FLAG-tagged Rad18 and lysed under condition A. FLAG-Rad18 and interacting proteins were analyzed by Western blotting. (C) MMS alters Rad18, not SHPRH, to promote the Rad18–SHPRH interaction. Purified GST-tagged Rad18 or SHPRH353–628 were used to pull down full-length FLAG-SHPRH or FLAG-Rad18 (respectively) from transfected cell lysates, which were mock treated or exposed to 0.005% MMS for 4 h. Associated proteins were analyzed by Western blotting.