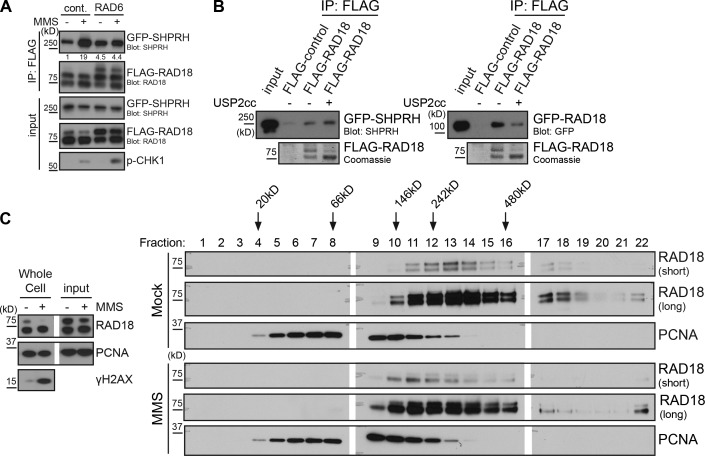
Figure 4.
Direct modulation of Rad18 ubiquitination affects Rad18–Rad18 and Rad18–SHPRH interactions. (A) Promoting Rad18 ubiquitination in vivo prevents damage-inducible binding to SHPRH. Cells were cotransfected with GFP-SHPRH, FLAG-Rad18, and either Rad6 or empty vector and then mock or MMS (0.005%) treated for 4 h before being lysed and processed as in Fig. 2 B. Quantification indicates ratio of GFP-SHPRH in the IP relative to the respective input sample, normalized to the mock-transfected, mock-treated control (cont.). (B) Deubiquitinating Rad18 affects its protein–protein interactions. FLAG-Rad18 was cotransfected with Rad6 and then purified from lysates under high-salt conditions. Beads were mock treated or deubiquitinated by Usp2 and used to pull-down GFP-SHPRH or GFP-Rad18 from transfected cells lysed as in Fig. 1 C. Pull-downs were analyzed by Western blotting. USP2cc, Usp2 catalytic core. (C) Rad18 shifts to a smaller complex after MMS treatment. Cells were mock treated or exposed to 0.01% MMS for 2 h and then lysed and separated on a 5–30% glycerol gradient. Gradient fractions were analyzed for the presence of Rad18 and PCNA by Western blotting. White lines indicate that intervening lanes have been spliced out.