Abstract
Arctostaphylos pungens “Manzanita” is an important shrub in the southwestern USA, and northern and central Mexico. Manzanita bears apple-like fruit that is utilised for a range of edible products. Over the past two years, several foliar disease problems were noted on this host in the San José de Gracia region of Mexico. The aim of the present study was to elucidate their identity through the analysis of morphological characters and DNA phylogeny (based on the large subunit nuclear ribosomal RNA gene and the ITS spacers and the intervening 5.8S rRNA gene of the nrDNA operon) of the fungi associated with these disease symptoms. Three species are newly described: Phaeococcomyces mexicanus sp. nov., a presumed epiphyte, and two species associated with leaf spots and defoliation, namely Coccomyces arctostaphyloides sp. nov. and Passalora arctostaphyli sp. nov. A fourth species is also associated with leaf spots and tip dieback is Harknessia arctostaphyli, for which an epitype is designated. All species can co-occur on the same shrub, which adds to the stress experienced by the plant, leading to further defoliation and dieback.
Keywords: Coccomyces, Harknessia, ITS, LSU, Passalora, Phaeococcomyces, systematics
INTRODUCTION
Arctostaphylos pungens (Ericaceae), or “Pointleaf Manzanita”, is native to the Southwestern USA and to northern and central Mexico. It grows in chaparral and woodland habitats, where it forms erect, spreading shrubs about 1–3 m in height (Márquez-Linares et al. 2005). The fruit is minute and apple-like, 5–8 mm diam, which is eaten by many birds and wildlife, and harvested for a multitude of uses. The common name “Manzanita” is Spanish for “little apple” referring to the small apple-shaped fruit. The fruit can be eaten raw or cooked, or is made into a type of jam in Mexico. Fruits may be dried and ground into a powder and then used as flavouring in soups. Leaves have also been used in the treatment of diarrhoea and to relieve itching and pain caused by poison oak and poison ivy. Tea made from the leaves and berries has also been used to treat bronchitis and urinary tract problems (Berg 1974, Weise et al. 1991).
Over the past two years, several severe foliar disease problems were noted on A. pungens plants growing in San José de Gracia region, Mexico (Fig. 1). Although not much is known about diseases of Arctostaphylos spp., the USDA website (nt.ars-grin.gov.) lists several plant pathogenic fungi as occurring on this host. Information about diseases linked to Arctostaphylos spp. occurring in Mexico, however, is sparse. The aim of the present study was therefore to characterise and identify the fungi associated with the various foliar disease symptoms found on this host.
Fig. 1.

A–C. Foliar disease symptoms leading to defoliation of Arctostaphylos pungens (Ericaceae) growing in the San José de Gracia region, Mexico.
MATERIALS AND METHODS
Isolates
Symptomatic leaves were placed in damp chambers for 1–2 d. Single conidial colonies were established from sporulating conidiomata on Petri dishes containing 2 % malt extract agar (MEA; Crous et al. 2009c) as described earlier (Crous et al. 1991). Colonies were sub-cultured onto potato-dextrose agar (PDA), oatmeal agar (OA), and MEA (Crous et al. 2009c), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Reference strains were deposited at the CBS-KNAW Fungal Biodiversity Centre (CBS) Utrecht, The Netherlands.
DNA isolation, amplification and analyses
Genomic DNA was extracted from fungal colonies growing on MEA using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA) according to the manufacturer’s protocol. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene, the first internal transcribed spacer, the 5.8S rRNA gene, the second ITS region and the 5’ end of the 28S rRNA gene (ITS). The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009a) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. The partial beta-tubulin gene sequence was generated as described in Lee et al. (2004). Additional sequences were obtained for comparison from the NCBI’s GenBank nucleotide database using megablast searches. The sequence alignment and subsequent phylogenetic analyses for the partial LSU sequences were carried out using methods described by Crous et al. (2006). Gaps longer than 10 bases were coded as single events for the phylogenetic analyses; the remaining gaps were treated as “fifth state” data. Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Morphology
Observations were made with a Zeiss V20 Discovery stereomicroscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an AxioCam MRc5 camera and software. Colony characters and pigment production were noted after 1 mo of growth on MEA, PDA and OA (Crous et al. 2009c) incubated at 25 °C. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970). Morphological descriptions were based on cultures sporulating on PDA. Scanning electron microscopy (SEM) was conducted by fixing small (2–3 mm3) sections of leaf tissue in 3 % glutaraldehyde with 0.1 M Sorensen phoshate buffer, pH 7.2 or 24 h. Afterwards, samples were rinsed three times with the Sorensen phosphate buffer for 1 min, and dehydrated with a series of ethanol solutions (30, 40, 50, 60, 70, 80, 90 and 100 %; the three last were used twice) for 15 min in every concentration. Leaf discs were placed in a dryer critical point CO2 Samdri-780A® (TOUSIMIS Research Corporation, Rockville, MD) at 31 °C and 1073 psi; discs were fixed on a 9 mm diam brass sampler with a copper conductive-adhesive tape and were covered with gold during 4 min in an ionizer JFC-1100® (Jeol, Tokyo). Samples were viewed with a JSM-35C® scanning electron microscope (Jeol) operated at 15 Kv in the Electronic Microscopy Laboratory of the Biology Department of the Autonomous University of Aguascalientes, Aguascalientes, Mexico.
RESULTS
Phylogeny
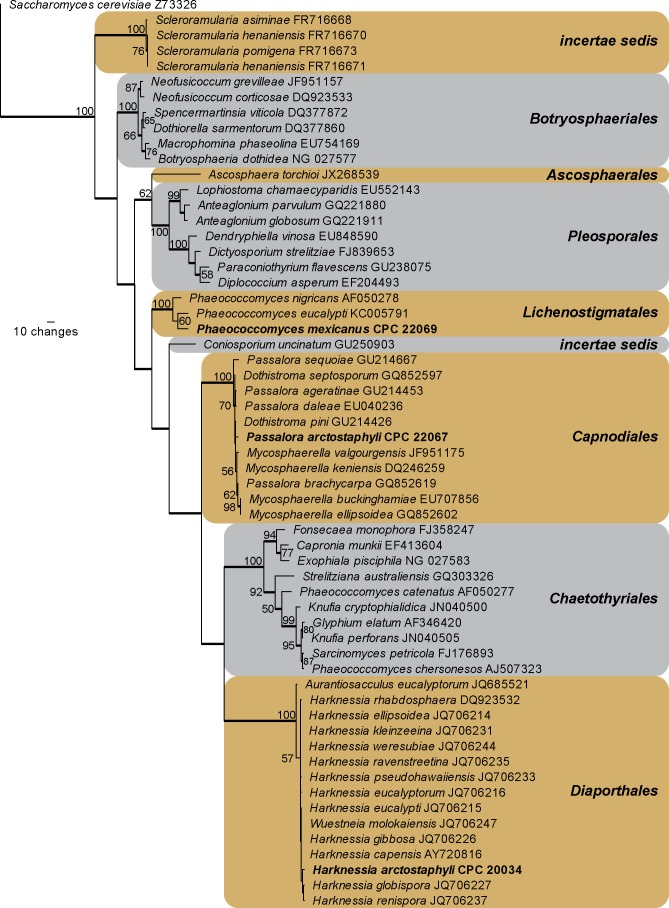
Amplicons of approximately 1 700 bases were obtained of the ITS region and the first approximately 900 bp of the LSU region for the isolates listed below. The LSU sequences were used to obtain additional sequences from GenBank, which were added to the alignment (Fig. 2) and the ITS to determine species identification (discussed in species notes where applicable). The manually adjusted LSU alignment comprised 59 sequences (including the outgroup sequence) and 806 characters including alignment gaps (available in TreeBASE) were used in the phylogenetic analysis; 327 of these were parsimony-informative, 39 were variable and parsimony-uninformative, and 440 were constant. Neighbour-joining analyses using three different substitution models (Uncorrected “p”, Kimura 2-parameter and HKY85) on the sequence alignment yielded trees with identical topologies to one another and support the same terminal clades as obtained from the parsimony analysis. The parsimony analysis of the LSU alignment yielded 662 equally most parsimonious trees (TL = 1209 steps; CI = 0.547; RI = 0.896; RC = 0.490), the first of which is shown in Fig. 2. The three taxa isolated in this study grouped in three distinct clades, namely Harknessia (Harknessiaceae, Diaporthales), the Dothistroma clade sensu Crous et al. (2009b) (Mycosphaerellaceae, Capnodiales) and a Phaeococcomyces s. str. clade (based on the inclusion of the type species of the genus, P. nigricans) with uncertain affinity compared to other “Phaeococcomyces” species clustering in Chaetothyriales.
Fig. 2.
The first of 662 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows ten changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Novel sequences generated for this study are shown in bold. Branches present in the strict consensus tree are thickened. Orders are shown in coloured blocks to the right of the tree. The tree was rooted to a sequence of Saccharomyces cerevisiae (GenBank accession Z73326).
TAXONOMY
Coccomyces arctostaphyloides O.M. Rico & Crous, sp. nov.
MycoBank MB808074
(Fig. 3)
Fig. 3.
Coccomyces arctostaphyloides (CBS H-21460). A, B. Leaf spot symptoms. C. Close-up of ascomata on leaf spot. D, F, G, H. Asci and paraphyses. E. Vertical section through ascoma, showing lateral flaps. I. Ascospores. J. Subcylindrical conidia with obtuse ends. Bars: A, B = 7 mm; C = 1 mm; E = 500 μm; D, F–J = 10 μm.
Etymology: Named after the host genus on which it occurs, Arctostaphylos.
Diagnosis: Asci (85–)120–150(–170) × (8–)12–16(–20) μm, cylindric-clavate, short-stalked, 6–8-spored, not staining in Meltzer’s iodine reagent. Ascospores (70–)80–90(–100) × (2.5–)3(–3.5) μm, tear-shaped, with non-persistant gelatinous sheath.
Type: Mexico: San José de Gracia, Sierra Fria, on leaves of Arctostaphylos pungens, 30 Nov. 2011, O. Moreno-Rico (CBS H-21460 – holotype).
Description: Leaf spots amphigenous, circular, (1–)7(–10) mm diam, centre pale grey with large black ascomata, outer zone brown, surrounded by a red-purple margin and yellow rim. Lesions begin as small circular necrotic spots (on the edges, apex or leaf centre), about 1 mm diam, enlarging up to 1 cm diam; leaf centres becomes grey with the development of apothecia; leaf veins become dark, and lesions are surrounded by yellow halo which can eventually cover the entire leaf, killing it. Ascomata amphigenous, black, circular, 500–1000 μm diam, opening by 3(–4) flaps; covering stroma 20–100 μm thick, lower stroma 10–50 μm thick. Asci cylindric-clavate, short-stalked, 6–8-spored, not staining in Meltzer’s iodine reagent, (85–)120–150(–170) × (8–)12–16(–20) μm. Ascospores filiform, tear-shaped, hyaline, aseptate, granular to guttulate, at times with gelatinous sheath (not persistant), (70–)80–90(–100) × (2.5–)3(–3.5) μm. Paraphyses filiform, extending above asci, hyaline, guttulate, up to 140 μm long, 2.5–3 μm wide, septate, cellular, covered in mucoid layer, tip curved, obtusely rounded, at times clavate, up to 4 μm diam. Conidiomata similar to ascomata in anatomy. Conidiophores lining inner cavity, 0–2-septate, subcylindrical, straight, branched, hyaline, 10–30 × 2–3 μm. Conidiogenous cells terminal and lateral, subcylindrical, hyaline, 10–17 × 2–2.5 μm; phialidic with periclinal thickening and prominent collarette. Conidia hyaline, smooth, subcylindrical, straight with obtuse ends, 4–7 × 1.5–2 μm.
Culture characteristics: In spite of several attempts and a range of different media (MEA, OA, PDA, synthetic nutrient poor agar), ascospores failed to germinate, which may be indicative of the biotrophic nature of this pathogen.
Notes: Coccomyces arctostaphyloides needs to be compared with C. arctostaphyli, which is known from Arctostaphylos in Europe (Eriksson 1970). The two pathogens are easily distinguished in that C. arctostaphyli has smaller, 4-spored asci (90–110 × 9–11 μm), and smaller ascospores (45–55 × 2–2.5 μm) (Eriksson 1970).
Harknessia arctostaphyli Cooke & Harkn., Grevillea 13: 111 (1885).
MycoBank MB218303
(Fig. 4)
Fig. 4.
Harknessia arctostaphyli (CBS H-21461). A. Dead and dying leaves. B. Leaf with tip blight. C. Oozing conidial mass (arrow). D–F. Conidia attached to conidiogenous cells. G. Conidia with basal appendages. Bars: C = 350 μm; all others = 10 μm.
Type: USA: California: San Francisco, Golden Gate Park, on leaves of Arctostaphylos sp., ex Harkness herb. ex Harkness Herbarium no. 2515 (BPI 368187 – holotype; BPI 798042 – isotype). Mexico: San José de Gracia, Sierra Fria, on leaves of Arctostaphylos pungens, 30 Nov. 2011, O. Moreno-Rico (CBS H-21461– epitype designated here, MBT177367; culture ex-epitype CBS 137228 = CPC 20034, GenBank ITS: KJ152781, LSU: KJ152784, TUB: KJ179923).
Description: Leaf spots associated with tip blight or leaf margins, dark brown, 1–3 mm diam, amphigenous with slightly raised border, eventually covering the whole leaf lamina; lesions initially with yellowish immature conidiomata, that become black with maturity; lesions gradually expand, leading to leaf necrosis, continuing down the petiole, causing blight and dieback. Description in vivo. Conidiomata amphigenous, pycnidioid, subepidermal, becoming erumpent, ovoid, black, up to 350 μm diam; dehiscence irregular with wide opening, border with pale yellow furfuraceous cells; wall of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the base of conidiomatal cavity. Conidiogenous cells lageniform to subcylindrical or ampulliform, hyaline, smooth, proliferating several times percurrently near apex, 5–10 × 4–7 μm. Macroconidia (19–)21–24(–25) × (10–)12(–13) μm, composed of a body with basal appendage; body brown, smooth, ellipsoid to ovoid, rarely apiculate, aseptate, with longitudinal striations covering restricted areas along the length of the conidium body, granular to guttulate, at times with central guttule. Basal appendage (18–)25–35(–45) × 2–4 μm, hyaline, tubular, smooth, thin-walled, flexuous, devoid of cytoplasm, at times walls collapsing. Microconidia not seen.
Culture characteristics: Colonies on MEA and PDA spreading, dirty white (surface and reverse) with moderate aerial mycelium and feathery margins, reaching 40 mm diam after 1 mo at 25 °C; colonies sporulating poorly on OA.
Notes: The Mexican collection closely matches the morphology of the holotype from California (see also Nag Raj 1993), and is therefore designated as epitype here. A blast search of the beta-tubulin sequences of our strains reveal highest identity to sequences of H. hawaiiensis [identities 407/421 (97 %), gaps 6/421 (1 %)] whereas the ITS sequence was the most similar to H. ravenstreetina [GenBank JQ706113; identities 416/421 (99 %), no gaps], H. globispora [GenBank JQ706105; identities 620/629 (99 %), gaps 4/629(0 %)], and H. eucalypti [GenBank JQ706089; identities 618/627 (99 %), gaps 2/627 (0 %)]. Morphologically H. arctostaphyli can easily be distinguished from these species based on the size of its ovoid to ellipsoid, longitudinally striate conidia, and the length of its basal appendages (Crous et al. 1993, 2012, Lee et al. 2004). Conidia of H. hawaiiensis are globose to broadly ellipsoidal, 9–12 × 8–11 μm, H. ravenstreetina are broadly ventricose, 14−20 × 7−9 μm, H. globispora are globose, 14–20 × 14–16 μm, and H. eucalypti are broadly ventricose, 19–18 × 11–15 μm.
Passalora arctostaphyli O. Moreno-Rico & Crous, sp. nov.
MycoBank MB808075
(Fig. 5)
Fig. 5.
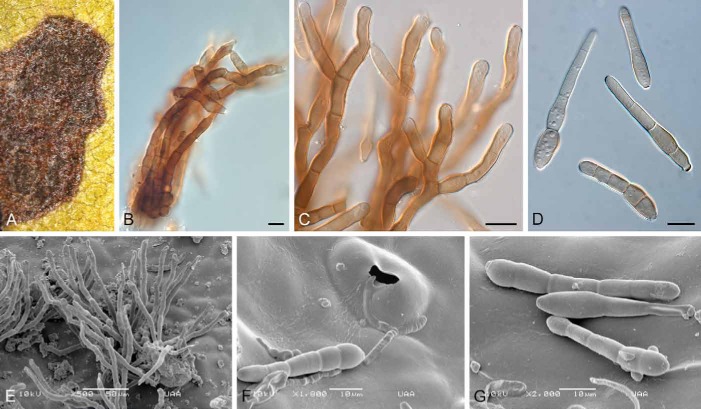
Passalora arctostaphyli (CBS H-21462). A. Leaf spot. B, C. Conidiophores in synnematous arrangement. D. Conidia. E. SEM photomicrograph of loosely arranged conidiophores. F. Conidium infection through a stoma. G. Conidia on leaf surface. Bars: B–D, F, G = 10 μm; E = 50 μm.
Etymology: Named after the host genus on which it occurs, Arctostaphylos.
Diagnosis: Conidiomata synnematous to fasciculate. Conidia (22–)45–60(–70) × (6–)7(–9) μm, solitary, brown, straight to gently curved, obclavate, 2–6-euseptate; hilum thickened and darkened, 3–4 μm diam.
Type: Mexico: San José de Gracia, Sierra Fria, on leaves of Arctostaphylos pungens, 30 Nov. 2011, O. Moreno-Rico (CBS H-21462 – holotype; culture ex-type CBS 137229 = CPC 22067, GenBank ITS: KJ152782, LSU: KJ152785).
Description: Leaf spots subcircular to irregular, with age becoming angular, as spots are then delimited by leaf veins; amphigenous, dark brown with a slightly raised border, 2–8 mm diam, at times with a thin, red-purple margin, and yellow halo; mature leaf spots coalesce, killing the leaves. Caespituli amphigenous, synnematous to fasciculate (up to 40 conidiophores per fascicle), forming on a well-developed, erumpent stroma, bursting through stomata, consisting of brown pseudoparenchymatal cells, up to 60 μm wide and 40 μm high. Conidiophores subcylindrical, dark brown, thick-walled, finely verruculose, branched or not, straight or curved, 4–25-septate, 60–200 × 5–8 μm. Conidiogenous cells integrated, terminal or as lateral branches on conidiophores, subcylindrical to clavate, with obtuse ends, brown, thick-walled, becoming thinner-walled at apex, finely verruculose, 10–35 × 5–7 μm; with multiple round scars, thickened, darkened, with visible central pore, 3–4 μm diam. Conidia (22–)45–60(–70) × (6–)7(–9) μm, solitary, brown, thin-walled, guttulate to granular, smooth to finely verruculose, straight to gently curved, obclavate, apex obtuse, base obconically truncate, 2–6-euseptate; hilum thickened and darkened, 3–4 μm diam.
Culture characteristics: Colonies erumpent with moderate aerial mycelium and feathery margins, reaching 7 mm diam after 1 mo on MEA and 15 mm diam on PDA; on MEA surface smoke-grey, reverse iron-grey. On PDA surface olivaceous grey, reverse iron-grey; colonies sterile.
Notes: Passalora arctostaphyli can be distinguished from Pseudocercospora gaultheriae (BPI 436613 – holotype; host originally misidentified as Arctostaphylos uva-ursi; Crous & Braun 2003), in that the latter has unthickened and not darkened conidial hila and scars, and so is a typical species of Pseudocercospora as circumscribed by Crous et al. (2013). Based on morphology, this collection is best accommodated in Passalora sensu Crous & Braun (2003). The ITS sequence of the Mexican strain retrieved several high similarity hits based on a megablast search of GenBank; these include Mycosphaerella valgourgensis [GenBank JF951152; identities 628/637 (99 %), gaps 1/637 (0 %)], Mycosphaerella microsora [GenBank EU167599; identities 667/678 (98 %), gaps 1/678 (0 %)], Mycosphaerella buckinghamiae [GenBank EU707856; identities 635/646 (98 %), gaps 1/646 (0 %)], and Passalora fulva [GenBank JQ768320; identities 539/551 (98 %), gaps 2/551 (0 %)].
Phaeococcomyces mexicanus O. Moreno-Rico & Crous, sp. nov.
MycoBank MB808076
(Fig. 6)
Fig. 6.
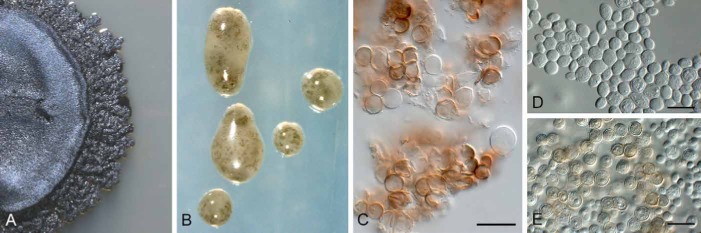
Phaeococcomyces mexicanus (CBS H-21463). A. Colony on PDA. B. Colony on SNA. C. Mature brown, verruculose, aseptate conidia. D, E. Immature, hyaline to pale brown conidia. Bars: C–E = 10 μm.
Etymology: Named after the country in which it was collected, Mexico.
Diagnosis: Colonies consisting of chlamydospore-like cells; cells aseptate, covered in mucus, globose, thick-walled, hyaline, becoming brown and verruculose, 5–8 μm diam.
Type: Mexico: San José de Gracia, Sierra Fria, on leaves of Arctostaphylos pungens, 30 Nov. 2011, O. Moreno-Rico (CBS H-21463 – holotype; culture ex-holotype CBS 137164 = CPC 22069, GenBank ITS: KJ152783, LSU: KJ152786).
Description: Colonies lacking mycelium but consisting of microsclerotia composed of a globular mass of chlamydospore-like cells; on host material these microsclerotia are loosely aggregated around conidiophores of Passalora arctostaphyli; cells aseptate, brown (hyaline when young), 5–8 μm diam, verruculose, covered in mucus, globose, thick-walled, catenulate, in acropetal chains expanding at colony margin; ellipsoid to globose, hyaline, thick-walled, covered in mucus, finely verruculose, 4–6 × 3–6 μm.
Culture characteristics: Colonies dense, with cells remaining attached to one another, dark brown to black on MEA and PDA, but on OA and SNA colonies form profuse amounts of mucus and appear looser with cells forming smaller clusters, and many globose conidia separate from one another; colonies erumpent, surface folded, stromatic, lacking aerial mycelium, and margins smooth, reaching 4–6 mm diam in the dark at 25 °C after 2 wk.
Notes: The ITS sequence of Phaeococcomyces mexicanus only retrieved distant hits based on a megablast search of GenBank; these include Phaeococcomyces nigricans [GenBank AF050278; identities 476/575 (83 %), gaps 32/575 (5 %)], P. eucalypti [GenBank KC005769; identities 472/574 (82 %), gaps 42/574 (7 %)], and even more distant Lasiodiplodia theobromae and L. crassispora [GenBank FJ478103 and EU918710; identities 320/380 (84 %), gaps 20/380 (5 %)], and Infundichalara microchona [GenBank FR667229; identities 331/396 (84 %), gaps 26/396 (6 %)]. In the LSU phylogeny, this strain is nestled in a Phaeococcomyces s. str. clade. Morphologically P. mexicanus resembles species of Phaeococcomyces, though the genus itself is polyphyletic, with species such as P. chersonesos belonging to the genus Knufia, and P. catenatus representing yet a third, as yet undescribed genus in this complex (Tsuneda et al. 2011).
DISCUSSION
This study has elucidated the taxonomy of several species occurring on leaves of Arctostaphylos pungens in Mexico. Other than Phaeococcomyces mexicanus, which appears to be an epiphyte occurring on leaf hairs of Arctostaphylos and conidiophores of Passalora arctostaphyli, several prominent foliar pathogens were encountered.
Although we were unable to cultivate Coccomyces arctostaphyloides in this study (despite numerous attempts with fresh material and a range of different media and incubation conditions), this is clearly a serious pathogen of Manzanita. The pathogen is consistently associated with prominent red, round leaf spots, which in some cases also led to defoliation. The genus Coccomyces presently includes close to 200 species, though very few have thus far been cultivated. Although limited molecular data are available, Coccomyces appears to be paraphyletic (Lantz et al. 2011).
The genus Harknessia (Harknessiaceae, Diaporthales) includes around 60 species that range ecologically from being endophytes, to saprobes, or foliar pathogens (Lee et al. 2004, Crous et al. 2012). Harknessia arctostaphyli appears to represent an important foliar pathogen of Manzanita, as it was consistently found to be associated with leaf tip blight symptoms, which in turn led to serious defoliation of infected shrubs.
Passalora arctostaphyli represents a new cercosporoid fungus on Manzanita, being associated with subcircular to irregular, dark brown leaf spots. Although no other cercosporoid fungi are presently known from this host, or genetically similar species were found in GenBank, some “Mycosphaerella” sexual morphs have been described from Arctostaphylos, namely M. minor and M. shawii. However, no reference has been found of any potential asexual morph, and no sexual morph was found on any of the diseased leaves we examined, or in the leaf litter. These observations led us to conclude that P. arctostaphyli probably represents a novel taxon. The generic circumscription of Passalora is, however, far from settled, as the type species, P. bacciligera, clusters apart from several clades that represent taxa identified as members of Passalora. A revision of the generic circumscriptions in this complex is presently in progress, and will be published shortly.
The genus Phaeococcomyces (based on P. nigricans, de Hoog 1977), includes species that exhibit colonies with globose to broadly ellipsoid budding cells and lack true hyphae. Phaeococcomyces mexicanus belongs to Phaeococcomyces s. str., while members that cluster in Chaetothyriales (P. catenatus, P. chersonesos, P. exophialae), were placed elsewhere, for example Knufia chersonesos (Tsuneda et al. 2011) and Exophiala exophialae (de Hoog et al. 2003), leaving only P. catenatus, which is a member of an as yet unnamed genus.
The fungi described in this study frequently occur together on the same plant. As some of them are clearly pathogenic, this co-occurrence results in increased host stress, leading to leaf and twig dieback, and defoliation of large portions of the shrubs (Fig. 1). Presently little is known about their ecology and distribution, highlighting the need for further research on these aspects.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Berg AR. (1974) Arctostaphylos Adans. In: Seeds of Woody Plants in the United States (Schopmeyer CS, ed.): 228–231 [Agricultural Handbook.] Washington, DC: USDA Forest Service; [Google Scholar]
- Crous PW, Braun U. (2003) Mycosphaerella and its Anamorphs. 1. Names published in Cercospora and Passalora. [CBS Biodiversity Series 1.] Utrecht: CBS-KNAW Fungal Biodiversity Centre; [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, Shin H-D, Nakashima C, Groenewald JZ. (2013) Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al (2009a) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. (2006) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ. (2009b) Unraveling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, Carnegie AJ, Groenewald JZ. (2012) A re-appraisal of Harknessia (Diaporthales), and the introduction of Harknessiaceae fam. nov. Persoonia 28: 49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009c) Fungal Biodiversity. [CBS Laboratory Manual Series 1.] Utrecht: CBS-KNAW Fungal Biodiversity Centre; [Google Scholar]
- Crous PW, Wingfield MJ, Nag Raj TR. (1993) Harknessia spp. occurring in South Africa. Mycologia 85: 108–118 [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991) Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Eriksson B. (1970) On Ascomycetes on Diapensiales and Ericales in Fennoscania. 1. Discomycetes. Symbolae Botanicae Upsalienses 19 (4): 1–71 [Google Scholar]
- Hoog GS de. (1977) Rhinocladiella and allied genera. Studies in Mycology 15: 1–140 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Vicente V, Caligiorne RB, Kantargliocu S, Tintelnot K, Gerrits van den Ende AHG, Haase G. (2003) Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. Journal of Clinical Microbiology 41: 4767–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz H, Johnston PR, Park D, Minter DW. (2011) Molecular phylogeny reveals a core clade of Rhytismatales. Mycologia 103: 57–74 [DOI] [PubMed] [Google Scholar]
- Lee S, Groenewald JZ, Crous PW. (2004) Phylogenetic reassessment of the coelomycete genus Harknessia and its teleomorph Wuestneia (Diaporthales), and the introduction of Apoharknessia gen. nov. Studies in Mycology 50: 235–252 [Google Scholar]
- Márquez-Linares MA, Jurado E, González-Elizondo S. (2005) Algunos aspectos de la biología de la manzanita (Arctostaphylos pungens HBK.) y su papel en el desplazamiento de bosques templados por chaparrales. Revista Ciencia UANL 9: 57–64 [Google Scholar]
- Nag Raj TR. (1993) Coelomycetous Anamorphs with Appendage-Bearing Conidia. Waterloo, ON: Mycologue Publications; [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Tsuneda A, Hambleton S, Currah RS. (2011) The anamorph genus Knufia and its phylogenetically allied species in Coniosporium, Sarcinomyces, and Phaeococcomyces. Botany 89: 523–536 [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise DR, Ward DE, Paysen TE, Koonce AL. (1991) Burning California Chaparral - an exploratory study of some common shrubs and their combustion characteristics. International Journal of Wildland Fire 1: 153–158 [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor SB. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds): 315–322 San Diego: Academic Press; [Google Scholar]