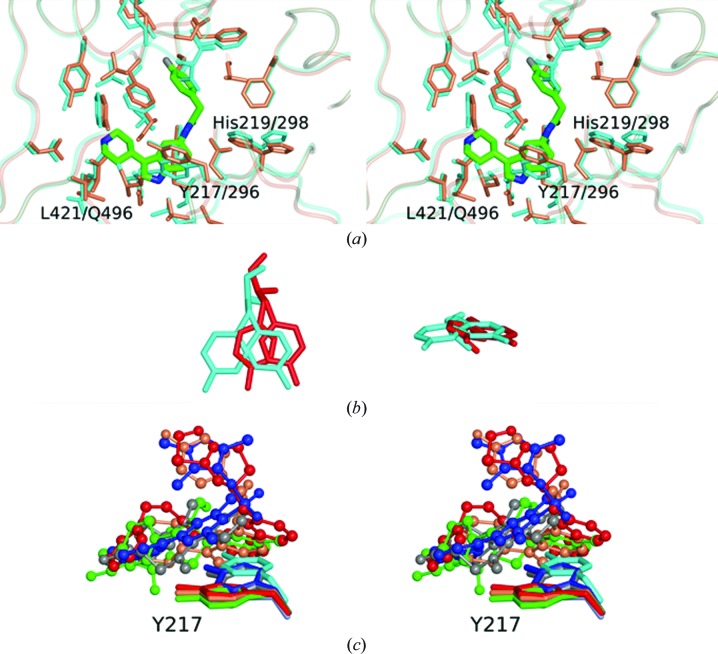
Figure 5.
Conformational flexibility of residue Tyr217. (a) Stereo overlay of the LmNMT–MyrCoA structure (coral) with HsNMT–MyrCoA (PDB entry 3iu1, cyan). The position of binding for the inhibitor 7AH is shown for reference. (b) Preferred positions of the LmNMT Tyr217 (red) and HsNMT Tyr296 (cyan) conformers. (c) Clustering of the various inhibitors. No inhibitor (HsNMT), cyan; DDD85646, blue; 6KV, grey; 7AH, coral; A6K, green; CWZ, red. For clarity, only the proximal 6KV molecule is shown. An inward orientation of the Tyr217 side chain prevails in the complexes with DDD85646 and 6KV, while outward orientations are observed in the complexes with 7AH, A6K and CWZ.