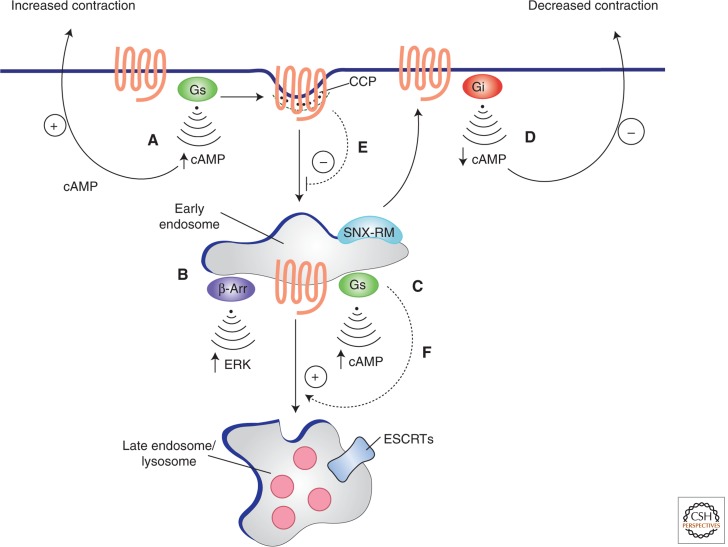
Figure 2.
Endocytosis modifies GPCR signaling and vice versa. (A) Activation of the β2-adrenergic receptor by catecholamine stimulates adenylyl cyclase through coupling to the heterotrimeric G protein Gs, thereby increasing the cAMP concentration in the cytoplasm. Receptor activation also initiates a process of regulated endocytosis of receptors, mediated by clathrin-coated pits, delivering activated receptors to early endosomes. (B,C) In endosomes receptors are thought to activate noncanonical signaling through the scaffold protein β-arrestin β-Arr, leading to activation of MAP kinases (B), and initiate a second “wave” of Gs-mediated activation of adenylyl cyclase that further increases cytoplasmic cAMP concentration (C). Receptors recycle to the PM by engaging a multiprotein sorting machinery, including sorting nexin 27, and the retromer complex (depicted by SNX-RM in the diagram). (D) On return to the PM, receptors couple to Gi, reducing adenylyl cyclase activity and decreasing cytoplasmic cAMP concentration. In cardiac muscle cells, this series of events causes an acute acceleration (Gs response) followed by deceleration (Gi response) of cardiac contraction (indicated by “+” and “−” arrows in figure). The GPCR-G protein system conversely regulates the endocytic process in at least two ways: (E) β-Adrenergic receptors locally prolong the surface lifetime of clathrin-coated pits that contain them, and (F) Gs acts in the early endosome membrane to promote endosome maturation through RAB5-to-7 conversion.