Figure 1.
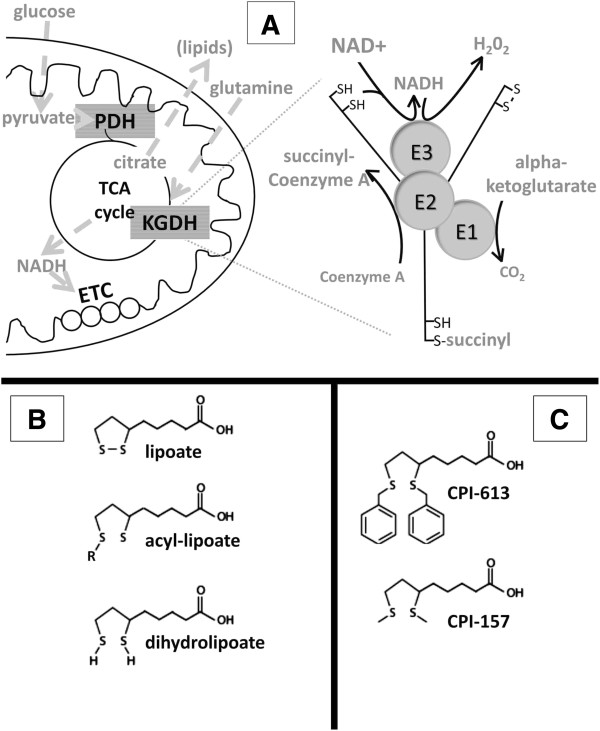
Role of α-ketoglutarate dehydrogenase and its lipoate residues. (A) On the left are selected details of mitochondrial metabolism, including the matrix TCA cycle and anabolic diversion of TCA citrate in support of cytosolic lipid biosynthesis. KGDH is an enzyme complex in the TCA cycle. Entry of glutamine-derived carbon into the TCA cycle is controlled by KGDH. Features of the structure and catalytic reactions of the KGDH complex are illustrated on the right. There are two sources of reducing potential for the catalysis of H2O2 production by E3: reduced lipoate (forward reaction) and NADH (reverse reaction). (B) Chemical details of the three intermediates in the natural lipoate catalytic cycles of KGDH and pyruvate dehydrogenase are shown. R indicates a succinyl residue in KGDH and an acetyl residue in pyruvate dehydrogenase. (C) Selected details of the structures of the two lipoate analogs used in these studies are shown. KGDH, α-ketoglutarate dehydrogenase; TCA, tricarboxylic acid.
