Figure 6. Carbamoyl Phosphate Synthase 1 (CPS1) is targeted for deglutarylation by SIRT5.
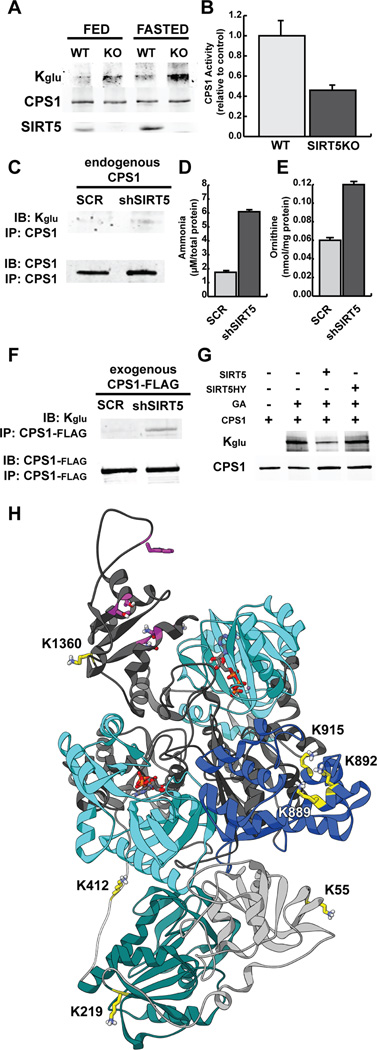
(A) CPS1 was immunoprecipitated from SIRT5 WT and KO mouse liver under fed and 48h fasted conditions and measured for glutarylation levels using an anti-Kglu antibody.
(B) CPS1 enzymatic activity was measured in hepatic lysates in SIRT5 WT and KO mice, and presented relative to WT control mice.
(C) CPS1 was immunoprecipitated from HeLa cells containing a shScramble (SCR) or shSIRT5 and measured for glutarylation levels using an anti-Kglu antibody.
(D) Ammonia levels from HeLa cells containing a shScramble (SCR) or shSIRT5 after 24h of serum starvation.
(E) Ornithine levels from HeLa cells containing a shScramble (SCR) or shSIRT5 after 24h of serum starvation.
(F) CPS1 was transfected in HEK293 cells containing a shScramble (SCR) or shSIRT5, immunoprecipitated, and measured for glutarylation levels using an anti-Kglu antibody.
(G) CPS1 was transfected in HEK293 cells and immunopurified. Glutarylation levels were measured using an anti-Kglu antibody in four samples: CPS1, glutarylated CPS1 that was chemically modified with glutaric anhydride (GA), glutarylated CPS1 after incubation with recombinant human SIRT5, glutarylated CPS1 after incubation with recombinant human SIRT5 HY mutant.
(H) A homology model of human CPS1 was built based on an E. coli CarA homologous region (light gray), encompassing an inactive glutamine amidotransferase domain (teal), and an E. coli CarB homologous region (dark gray) containing two ATP grasp domains (cyan), an oligomerization domain (blue), and a NAG-binding site (magenta). Glutarylated lysine residues targeted for SIRT5 removal are labeled and highlighted in yellow.
