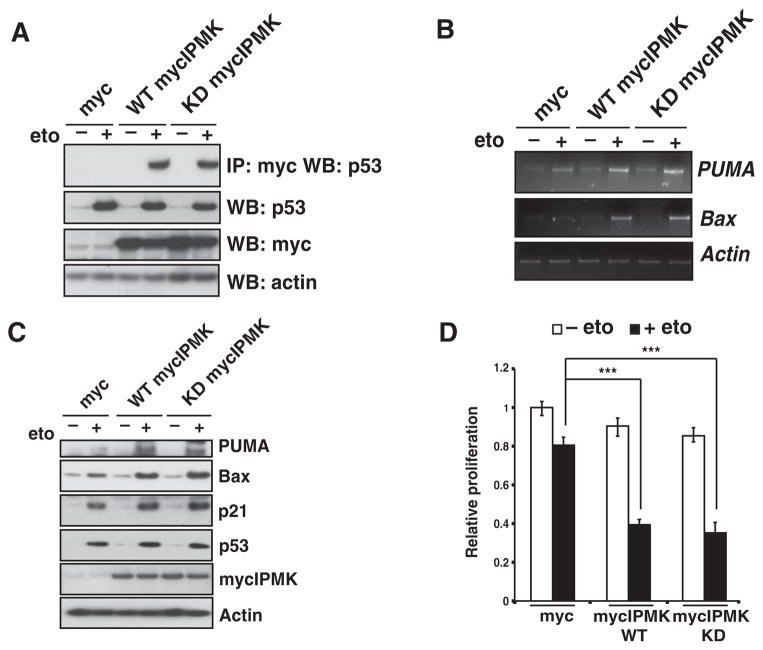
Fig. 7.
IPMK coactivation of p53 transcriptional activity is kinase-independent. (A) Immunoprecipitation and Western blotting were used to detect binding between p53 and IPMK in lysates from U2OS cells transfected with plasmids encoding wild-type (WT) mycIPMK and kinase-deficient (KD) mycIPMK constructs and treated with 20 μM etoposide. Blots are representative of three experiments. (B and C) Detection of PUMA and Bax mRNAs (B) and proteins (C) in transfected, etoposide-treated U2OS cells. Blots are representative of three experiments. (D) Cell proliferation of transfected, etoposide-treated U2OS cells. Data are means ± SEM from three experiments. ***P < 0.001, one-way analysis of variance (ANOVA).