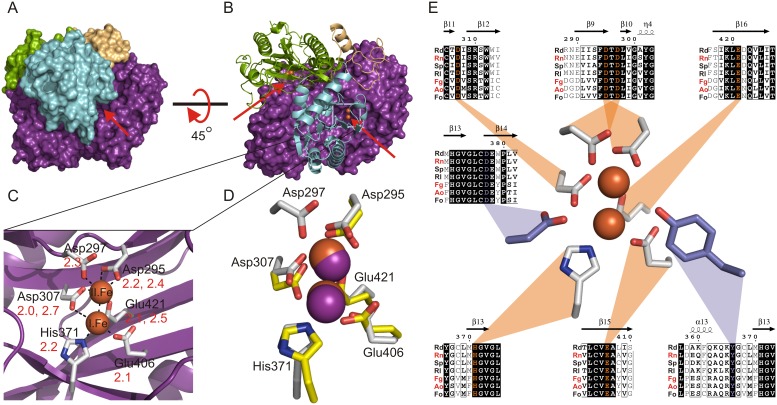
Figure 4. Dimerization shapes the tunnel active sites of RdDddP with a binuclear iron metal center that is conserved in DddP lyases.
(a) The dimer with one monomer in a cartoon plot presentation (beige, blue, green from the N-to the C-terminus), the second monomer in purple and the iron ions colored in orange. (b) The entrance to the two active sites and their path is indicated by the red arrows. (c) The metal binding site of RdDddP with the two iron ions in orange and the conserved metal coordinating residues in grey. The distances between residues and metal ions, in angstroms, are indicated in red. (d) Comparison of the RdDddP metal binding site (grey, iron ions as orange spheres) with the metal binding site of the methionine aminopeptidase (1MAT), (yellow sticks, cobalt ions as purple spheres). (e) Conservation of metal binding site and the putative catalytic residues in diverse DddP lyases from marine bacteria and fungi. Rd: Roseobacter denitrificans OCh 114, Rn: Roseovarius nubinhibens ISM, Sp: Silicibacter pomeroyi DSS-3, Rl: Roseobacter litoralis Och 149, Fg: Fusarium graminearum cc19, Ao: Aspergillus oryzae RIB40. Fc: Fusarium culmorum. Abbreviations in red indicate functionally characterized DddP lyases based on Todd et al. 2009. (notably here only a subset of DddP lyases is shown and the residues for metal binding and proposed catalysis are invariant in all DddP lyases presented in Todd et al. 2009). The alignment was prepared with ClustalW.