Abstract
The presence of pharmaceutical products in the aquatic environment has been reported in several studies. However, the impact of these drugs on living organisms is still uncharacterized. Here, we investigated the effects of acute exposure to either diazepam or fluoxetine on the stress response in Danio rerio. We showed that diazepam and fluoxetine inhibited the stress axis in zebrafish. Intermediate concentrations of diazepam suppressed the stress response as measured by cortisol levels, whereas fluoxetine inhibited cortisol increase at concentrations similar to those found in the environment. These data suggest that the presence of psychoactive drugs in aquatic ecosystems could cause neuroendocrine dysfunction in fish.
Introduction
The presence of pharmaceutical drugs in the aquatic environment is a significant concern to regulatory agencies because these drugs could affect both the human population and aquatic ecosystems. Benzodiazepines and selective serotonin reuptake inhibitors (SSRIs) are present in wastewater effluent and are neither cleared nor photobleached after treatment of the effluents [1]–[3]. The environmental concentrations of these drugs range from 0.04 to 0.88 µg/L for diazepam [4]–[6] and 0.012 to 1 µg/L for fluoxetine [4], [7]–[10]. Benzodiazepines and SSRIs exert anxiolytic effects and can interfere with neuroendocrine stress axis activity [11]–[14]. Although these drugs have been detected in an extensive variety of environments, there is little information regarding the effects of these compounds in living organisms [15].
The stress response system helps the individuals to deal with adverse conditions [16]. For instance, increases in cortisol levels during stress can lead to hyperglycemia, which could provide energy for defensive actions [17]–[19], and also participate of the osmoregulation processes in fish [20]. Thus, the harmful effects of pollutants on the fish stress response can adversely affect their survival [21]–[22], since both drugs can interfere with stress response in humans [23]–[25]. In this context, we hypothesized that the concentrations of diazepam and fluoxetine in the environment can interfere with the stress response in fish. We tested this possibility using zebrafish (Danio rerio) as the experimental model. This fish species has many advantages as a model organism because of its easy handling and maintenance as well as its genetic homology with humans [26]–[28]. Recent studies have reinforced the use of the zebrafish model for stress research [28]–[35].
Materials and Methods
Ethical note
This study was approved by the Ethics Commission for Animal Use (CEUA) at Universidade de Passo Fundo, UPF, Passo Fundo, RS, Brazil (Protocol #7/2013-CEUA) and met the guidelines of Conselho Nacional de Controle de Experimentação Animal (CONCEA).
Animals
A stock population of 1188 mixed-sex, adult wild-type zebrafish (Danio rerio) of the short-fin (SF) strain were held in 2 tanks with constant aeration and equipped with biological filtering under a natural photoperiod (approximately 14 h light: 10 h dark). Water was maintained at 26±2°C and pH 7.0±0.25, with dissolved oxygen levels at 6.5±0.4 mg/L, total ammonia levels at 0.01 mg/L, total hardness at 6 mg/L, and alkalinity at 22 mg/L CaCO3.
Experimental design
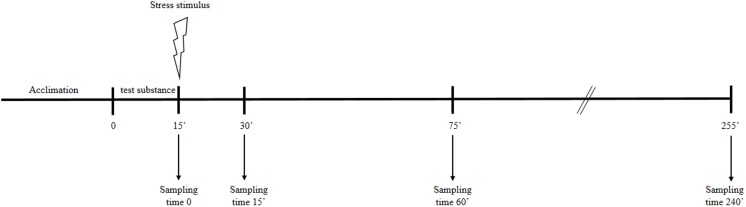
For each test substance (fluoxetine or diazepam), fish from the stock population were distributed in 32 glass aquaria (30×30×30 cm, six fish per tank), acclimatized for seven days and fed with commercial food flakes (TetraMin, Tetra, Melle, Germany). Twenty-four hours later, fish were exposed to the test substance for 15 minutes. Animals were then submitted to a stress stimulus, consisting of chasing fish with a net for two minutes was applied [30], and sampled after 0, 15, 60 and 240 minutes for whole body cortisol analysis (Fig. 1). Similarly, groups were submitted to test substance without stress test (sampled at the same time points), aiming to evaluate an eventual stress effect of the substance per se. A basal situation, i.e. without drug exposure and stress test was performed as control.
Figure 1. Schematic view of the experimental design of the study.
The test substance refers to the control (no substance), diazepam (0.88, 16, or 160 µg/L) or fluoxetine (1, 25 or 50 µg/L).
This setup was replicated 3 times. For whole-body cortisol determination, pools of 2 fish (to obtain approximately 0.5 g of tissue) were examined, with a total of 6 pools of 2 fish for each treatment and time point.
Diazepam (União Química, 5 mg/ml) was used at the following three concentrations: 0.88 µg/L, which is the highest detected environmental concentration [5]; 16 µg/L, which is 10% of the concentration that promotes behavioral effects; and 160 µg/L, which is the concentration with reported effects in zebrafish behavior [36].
Fluoxetine (Daforin EMS, oral solution, 20 mg/mL) was tested at concentrations of 1 µg/L [7], 25 µg/L and 50 µg/L (25 and 50 times the environmental concentration, respectively).
Cortisol extraction and analysis
Fish were captured and immediately frozen in liquid nitrogen for 10–30 s, followed by storage at −20°C until cortisol extraction. Whole-body cortisol was extracted using the method described by Oliveira et al. [30]. The accuracy was tested by calculating the recoveries from samples spiked with known amounts of cortisol (50, 25 and 12.5 ng/mL). The mean detection of spiked samples was 94.3%. All of the cortisol values were adjusted for recovery with the following equation: cortisol value = measured value × 1.0604.
Tissue extracts were resuspended in 1 mL PBS, and whole-body cortisol levels were measured in duplicate for each extraction using a commercially available enzyme-linked immunosorbent assay kit (EIAgen CORTISOL test, BioChem ImmunoSystems). This kit was fully validated for zebrafish tissue extracts using the methodology described by Sink et al. [37]. Precision was tested by performing 12 repeated assays on seven randomly chosen samples on the same 96-weel plate and calculating the intra-assay coefficient of variation (CV). Reproducibility was tested by assaying the same samples on different plates and calculating the inter-assay CV. To test for linearity and parallelism, the tissue extracts underwent serial dilutions in the buffer provided with the kit. A strong positive correlation between the curves was observed (R2 = 0.9108), and it was determined that the samples had low inter- and intra-assay CV values (7–10% and 5–9%, respectively).
Statistics
Homogeneity of variance was determined using Hartley's test, and normality was determined using the Kolmogorov-Smirnov test. Whole-body cortisol concentrations were compared using a two-way ANOVA, with drug concentration and time after the stressor as the independent variables, followed by a Bonferroni post-test. Differences with p values <0.05 were considered to be statistically significant.
Results
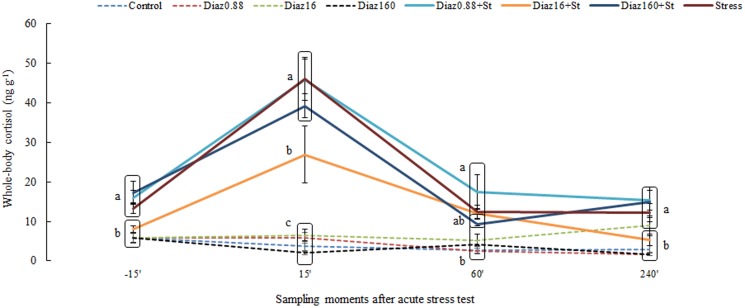
There were a significant interaction between drug concentration and time after stress induction (P<0.0001; F3,21 = 9.086). Fish exposed to 16 µg/L of diazepam and submitted to the acute stress test (Fig. 2) had a reduced cortisol response to an acute stressor at 15 and 240 minutes after stress. Any effect on cortisol profiles were detected on the time course curve, in fish exposed only to diazepam without acute stress test.
Figure 2. Whole-body cortisol concentrations in zebrafish to diazepam followed by an acute stress test and respective controls.
The values are expressed as the mean ± standard error of mean. Different small letters indicate significant group differences in each sampling time.
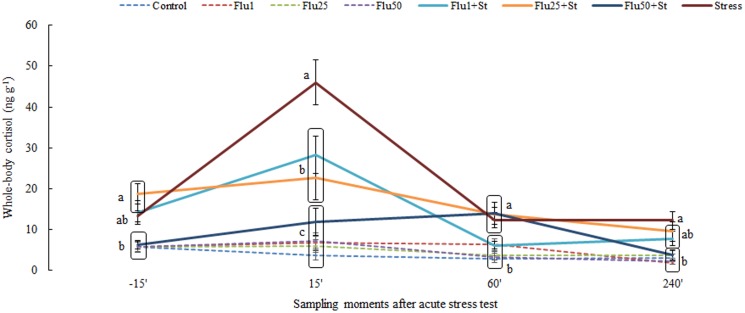
For fluoxetine exposure (Fig. 3), we found a significant interaction between drug concentration and time after stress induction (P<0.0001; F3,21 = 7.492). Exposure only to the three fluoxetine concentrations did not cause any effect on whole-body cortisol while the combination of drug exposure and stress test resulted in an impaired cortisol response to an acute stressor at 15 minutes compared to the stressed only fish, with the highest concentration (50 µg/L) causing the strongest inhibitory effect. However, these fish (Flu 50 µg/L+stress group) had lower pre-stress concentrations compared to groups Flu 1 µg/L+stress and 25 µg/L+stress.
Figure 3. Whole-body cortisol concentrations in zebrafish to fluoxetine followed by an acute stress test and respective controls.
The values are expressed as the mean ± standard error of mean. Different small letters indicate significant group differences in each sampling time.
Discussion
We show that acute exposure to diazepam and fluoxetine diluted in water impair the stress axis function, as drug-exposed fish had lower cortisol levels than control fish when exposed to an acute stress test. Both benzodiazepines and SSRIs possess anxiolytic activity [2], [14], [25], [36], and it is reasonable to suggest that these deleterious effects on the stress axis were related to a central action of these drugs.
The mechanism by which these drugs reduce the stress response may be directly related to the hypothalamus and/or pituitary gland, likely without the involvement of interrenal tissue. The serotonergic system of the brain plays a key role in autonomic, neuroendocrine and behavioral integration of the stress response in fish as well as mammals [38]. Benzodiazepines have been shown to influence the activity of the hypothalamus-pituitary-adrenal axis in humans by reducing basal adrenocorticotropic hormone (ACTH) and cortisol release after acute administration [39]–[41].
Our results showed that only the intermediate concentration of diazepam (16 µg/L) altered cortisol levels after stress induction. Other concentrations did not affect the cortisol stress response, demonstrating that the concentration of diazepam in the environment [5] was insufficient to impair the stress axis. Surprisingly, the highest concentration used (160 µg/L) did not alter cortisol response, suggesting diazepam may have a U-shaped dose-response curve similar to the effects of alcohol on fish behavior [30], [39] and stress response [42]. Similar curve pattern was found in cortisol effects on human memory [43].
However, acute exposure to all of the tested concentrations of fluoxetine impaired the cortisol response to acute stress, including the concentration identified in the environment (1 µg/L).
Although the anxiolytic effect exerted by diazepam and fluoxetine is well understood, the exact mechanisms by which these drugs block the biological response of cortisol in response to stress are still unclear. A fish with an impaired stress response loses its ability to maintain homeostasis against stressors by reducing the ability to promote ionic, metabolic and behavioral adjustments necessary for the stress response [22], [31]–[33]. A fish unable to display a normal cortisol response shows a reduced ability to respond to ongoing challenges posed by aquaculture [40]. Additionally, increased activity and boldness as well as reduced sociability in zebrafish that came in contact with these substances may also increase the risk of predation [41], [44], making the consequences of environmental contamination with these drugs difficult to predict.
The effects of diazepam and fluoxetine on cortisol levels have been previously reported in other species. For example, diazepam has been shown to decrease plasma cortisol levels, especially in humans experiencing stress [45]. Another study in elephant seals (Mirounga angustirostris) found that when animals were sedated with a combination of drugs ([tiletamine hydrochloride 50 mg/mL, zolazepam hydrochloride 50 mg/mL], ketamine, and diazepam) in the field, they did not exhibit a cortisol stress response. The use of this drug combination appears to decrease the responsiveness of the animals to handling and subsequent stress [46]. A similar result was observed in a study with Weddell seals (Leptonychotes weddellii), as treatment with diazepam improved the cortisol response of handled animals [47]. With regard to fluoxetine, chronic exposure to this drug decreased the cortisol levels in zebrafish in the novel tank test [28], [29]. Therefore, despite the differences in protocols and models, we have shown for the first time that animals exposed to either diazepam or fluoxetine diluted in water have a blunted cortisol response when exposed to a highly stressful situation.
Our results highlight that the presence of anxiolytic drugs in aquatic ecosystems may promote ecologically important but undervalued effects. It is imperative that new testing protocols be developed to examine the environmental impact of waste pharmaceuticals on fish and other aquatic life. Together with previous research, our data indicate that zebrafish are sufficient model organisms for studying pharmaceutical pollutants and their impact on the environment.
Funding Statement
LJGB and ALP have a CNPq research fellowship (302073/2011-6 and 472715/2012-7, respectively). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Calisto V, Domingues MRM, Esteves VI (2011) Photodegradation of psychiatric pharmaceuticals in aquatic environments e Kinetics and photodegradation products. Water Res 45: 6097–6106. [DOI] [PubMed] [Google Scholar]
- 2.Brodin T, Fick J, Jonsson M, Klaminder J (2013) Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural Populations. Science 339, 814. [DOI] [PubMed]
- 3. Gunnarson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DG (2008) Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ Sci Technol 42: 5807–5813. [DOI] [PubMed] [Google Scholar]
- 4. Calisto V, Esteves VI (2009) Psychiatric pharmaceuticals in the environment. Chemosphere 77: 1257–1274. [DOI] [PubMed] [Google Scholar]
- 5. Ternes T, Bonerz M, Schmidt T (2001) Determination of neutral pharmaceuticals in wastewater and rivers by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 938: 175–185. [DOI] [PubMed] [Google Scholar]
- 6. Calamari D, Zuccato E, Castiglioni S, Bagnati R, Fanelli R (2003) Strategic Survey of Therapeutic Drugs in the Rivers Po and Lambro in Northern Italy. Environ Sci Technol 37: 1241–1248. [Google Scholar]
- 7. Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, et al. (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ Sci Technol 36: 1202–1211. [DOI] [PubMed] [Google Scholar]
- 8. Metcalfe CD, Miao XS, Koenig BG, Struger J (2003) Distribution Of Acidic And Neutral Drugs In Surface Waters Near Sewage Treatment Plants In The Lower Great Lakes, Canada. Environm Toxicol Chem 22: 2881–2889. [DOI] [PubMed] [Google Scholar]
- 9. Schultz MM, Furlong ET (2008) Trace Analysis of Antidepressant Pharmaceuticals and Their Select Degradates in Aquatic Matrixes by LC/ESI/MS/MS. Anal Chem 80: 1756–1762. [DOI] [PubMed] [Google Scholar]
- 10. Togunde OP, Oakes KD, Servos MR, Pawliszyn J (2012) Determination of Pharmaceutical Residues in Fish Bile by Solid- Phase Microextraction Couple with Liquid Chromatography-Tandem Mass Spectrometry (LC/MS/MS). Environ Sci Technol 46: 5302–5309. [DOI] [PubMed] [Google Scholar]
- 11. Oggier DM, Weisbrod CI, Stoller AM, Zenker AK, Fent K (2010) Effects of diazepam on gene expression and link to physiological effects in different life stages in zebrafish Danio rerio . Environ Sci Technol 44: 7685–7691. [DOI] [PubMed] [Google Scholar]
- 12. Park JW, Heah TP, Gouffon JS, Henry TB, Sayler GS (2012) Global gene expression in larval zebrafish (Danio rerio) exposed to selective serotonin reuptake inhibitors (fluoxetine and sertraline) reveals unique expression profiles and potential biomarkers of exposure. Environm Pollut 167: 163–170. [DOI] [PubMed] [Google Scholar]
- 13. Barbosa Junior A, Alves FL, Pereira ASF, Ide LM, Hoffmann A (2012) Behavioral characterization of the alarm reaction and anxiolytic-like effect of acute treatment with fluoxetine in piauçu fish. Phys Behav 105: 784–790. [DOI] [PubMed] [Google Scholar]
- 14. Bencan Z, Sledge D, Levin ED (2009) Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav 94: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santos LH, Araújo AN, Fachini A, Pena A, Delerue-Matos C, et al. (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater175: 45–95. [DOI] [PubMed] [Google Scholar]
- 16. Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integ Comp Biol 42: 517–525. [DOI] [PubMed] [Google Scholar]
- 17. Eames SC, Philipson LH, Prince VE, Kinkel MD (2010) Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish 7: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Vuren JHJ, Hattingh J (1978) A seasonal study of the haematology of wild freshwater fish. J Fish Biol 13: 305–313. [Google Scholar]
- 19. Groff JM, Zinkl JG (1999) Hematology and clinical chemistry of cyprinid fish. Common carp and goldfish. Vet Clin North Am Exot Anim Pract 2: 741–776. [DOI] [PubMed] [Google Scholar]
- 20. Mancera MJ, Carrión RL, Martin DP, Rio DM (2002) Osmorresgulatory action of PRL, GH and cortisol in the gilthead seabrem (Sparus aurata L). Gen Comp Endocrinol 129: 95–103. [DOI] [PubMed] [Google Scholar]
- 21. Cericato L, Neto JGM, Fagundes M, Kreutz LC, et al. (2008) Cortisol response to acute stress in jundiá Rhamdia quelen acutely exposed to sub-lethal concentrations of agrichemicals. Comp Biochem Physiol C 148: 281–286. [DOI] [PubMed] [Google Scholar]
- 22. Cericato L, Neto JGM, Kreutz LC, Quevedo RM, et al. (2009) Responsiveness of the interrenal tissue of Jundiá (Rhamdia quelen) to an in vivo ACTH test following acute exposure to sublethal concentrations of agrichemicals. Comp Biochem Physiol C 149: 363–367. [DOI] [PubMed] [Google Scholar]
- 23. McIntyre IM, Norman TR, Burrows GD, Armstrong SM (1993) Alterations to plasma melatonin and cortisol after evening alprazolam administration in humans. Chronobiol Int 10: 205–213. [DOI] [PubMed] [Google Scholar]
- 24. Osman OT, Hsiao JK, Potter WZ (1993) Dose-dependent effects of intravenous alprazolam on neuroendocrine, biochemical, cardiovascular, and behavioral parameters in humans. Psychopharmacology 111: 295–300. [DOI] [PubMed] [Google Scholar]
- 25. Curtis GC, Abelson JL, Gold PW (1997) Adrenocorticotropic hormone and cortisol responses to corticotropin-releasing hormone: changes in panic disorder and effects of alprazolam treatment. Biol Psychiatry 41: 76–85. [DOI] [PubMed] [Google Scholar]
- 26. Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, et al. (2000) The synthetic relationship of the zebrafish and human genomes. Genome Res 10: 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldsmith P (2004) Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol 4: 504–12. [DOI] [PubMed] [Google Scholar]
- 28. Egan R, Bergner CL, Hart PC, Cachat JM, Canavello PR, et al. (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, et al. (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Prot 5(11): 1786–1799. [DOI] [PubMed] [Google Scholar]
- 30. Oliveira TA, Koakoski G, Kreutz LC, Ferreira D, et al. (2013) Alcohol Impairs Predation Risk Response and Communication in Zebrafish. PLOSONE 8(10): e75780 doi:10.1371/journal.pone.0075780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, Silva LB, et al. (2007) Wholebody cortisol increases after direct and visual contact with the predator in zebrafish, Danio rerio . Aquaculture 272: 774–778. [Google Scholar]
- 32. Barcellos LJG, Ritter F, Kreutz LC, Cericato L (2010) Can Zebrafish Danio rerio learn about predation risk? The effect of a previous experience on the cortisol response in subsequent encounters with a predator. J Fish Biol 76: 1032–1038. [Google Scholar]
- 33. Barcellos LJG, Volpato GL, Barreto RE, Coldebella I, Ferreira D (2011) Chemical communication of handling stress in fish. Phys Behav 103: 372–375. [DOI] [PubMed] [Google Scholar]
- 34. Piato AL, Capiotti KM, Tamborski A, Oses JP, Barcellos LJG, et al. (2011) Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Progr Neuro-Psychopharm Biol Psychiatry 35: 561–567. [DOI] [PubMed] [Google Scholar]
- 35. Dal Santo G, Conterato GMM, Barcellos LJG, Rosemberg DB, Piato AL (2013) Acute restraint stress induces an imbalance in the oxidative status of the zebrafish brain. Neurosci Lett 558: 103–108. [DOI] [PubMed] [Google Scholar]
- 36. Gebauer DL, Pagnussat N, Piato AL, Schaefer IC, Bonan CD, et al. (2011) Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol Biochem Behav 99: 480–486. [DOI] [PubMed] [Google Scholar]
- 37. Sink TD, Lochmann RT, Fecteau KA (2007) Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu and golden shiners. Fish Physiol Biochem 75: 165–171. [DOI] [PubMed] [Google Scholar]
- 38. Winberg S, Nilsson A, Hylland P, et al. (1997) Serotonin as a regulator of hypothalamic-pituitary-interrenal activity in teleost fish. Neurosci Lett 230: 113–116. [DOI] [PubMed] [Google Scholar]
- 39. Gerlai R, Lahav M, Guo S, Rosenthal A (2000) Drinks like a fish: zebrafish (Danio rerio) as abehavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67: 773–82. [DOI] [PubMed] [Google Scholar]
- 40. Brodeur JC, Sherwood G, Rasmussen JB, Hontela A (1997) Impaired cortisol secretion in yellow perch (Perca flavescens) from lakes contaminated by heavy metals: in vivo and in vitro assessment, Can J Aquat Sci. 54: 2752–2758. [Google Scholar]
- 41. Dugatkin LA (1992) Tendency to inspect predators predicts mortality risk in the guppy (Poecilia reticulata). Behav Ecol 3: 124–127. [Google Scholar]
- 42. Barreto RE, Volpato GL (2004) Caution for using ventilatory frequency as an indicator of stress in fish. Behav Proces 66: 43–51. [DOI] [PubMed] [Google Scholar]
- 43. Schilling TM, Kölsch M, Larra MF, Zech CM, Blumenthal TD, et al. (2013) For whom the bell (curve) tolls: Cortisol rapidly affects memory retrieval by an inverted U-shaped dose-response relationship. Psychoneuroendocrinol 38: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 44. Krause J, Ruxton GD (2002) Living in Groups. Oxford University Press. London 2002: 210p. [Google Scholar]
- 45. Pomara N, Willoughby LM, Sidtis JJ, Cooper TB, Greenblatt DJ (2005) Cortisol response to diazepam: its relationship to age, dose, duration of treatment, and presence of generalized anxiety disorder. Psychopharmacology 178: 1–8. [DOI] [PubMed] [Google Scholar]
- 46. Champagne CD, Houser DS, Costa DP, Crocker DE (2012) The effects of handling and anesthetic agents on the stress response and carbohydrate metabolism in northern elephant seals. PLoS One. 2012 7(5): e38442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harcourt RG, Turner E, Hall A, Waas JR, Hindell M (2010) Effects of capture stress on free-ranging, reproductively active male Weddell seals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196: 147–54. [DOI] [PubMed] [Google Scholar]