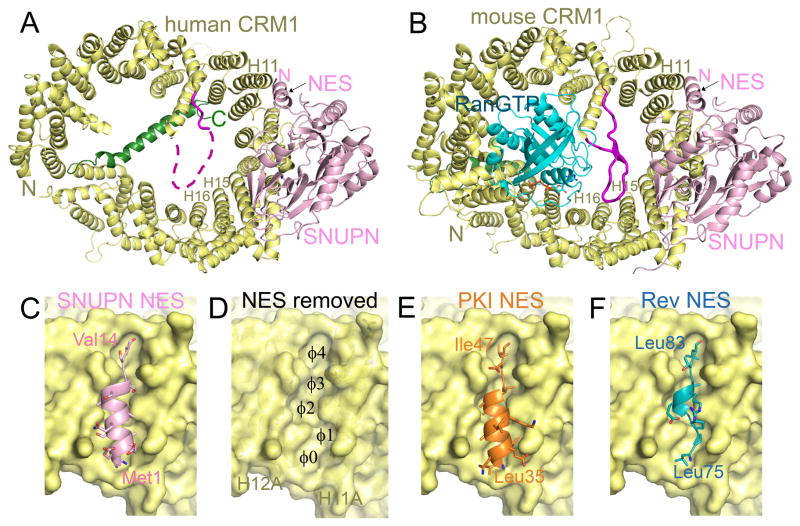
Figure 3. Cargo-bound CRM1 complexes.
A) Structures of the same human CRM1-SNUPN and B) mouse CRM1-RanGTP-SNUPN complexes in Fig 2, but shown here with more details to explain CRM1-cargo recognition. Surface representations of the NES binding groove of the mouse CRM1-RanGTP-SNUPN complex, C) with the SNUPN NES bound, D) the NES peptide removed to view the underlying Φ0-4 pockets in the groove, E) the PKI NES bound (3NBY) and F) the HIV-1 Rev-NES bound (3NBZ). NES peptides are shown in cartoon with their side chains in sticks. The three peptides have diverse amino acid sequences and adopt different secondary structures to accommodate their hydrophobic side chains into the same Φ0-4 hydrophobic pockets.