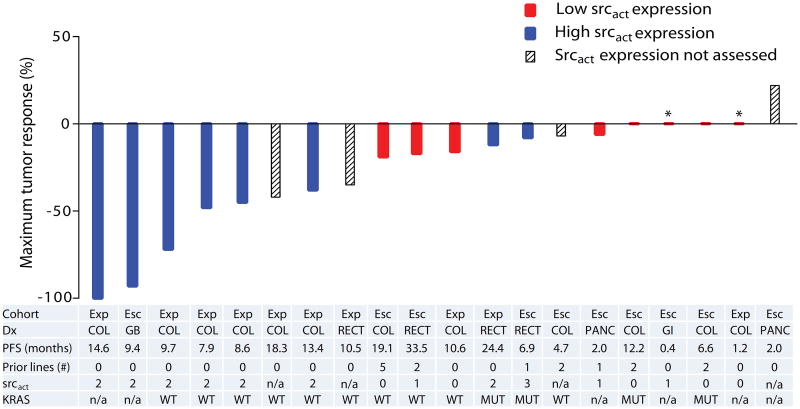
Figure 3. Clinical activity of dasatinib, capecitabine, oxaliplatin, and bevacizumab.
* Clinical progression prior to first restaging
Best response by srcact status for 20 evaluable subjects. Cohort: Exp (expansion), Esc (dose escalation); Dx (Diagnosis): COL (colon cancer), GB (gallbladder cancer), GI (adenocarcinoma of the small bowel), PANC (pancreatic cancer), RECT (rectal cancer); Prior lines (number of prior lines of treatment for metastatic disease); srcact (activated src expression): 0=none; 1= weak/faint; 2= moderate; 3= strong/circumferential; KRAS: n/a (not assessed), MUT (mutation detected), WT (no mutation detected).