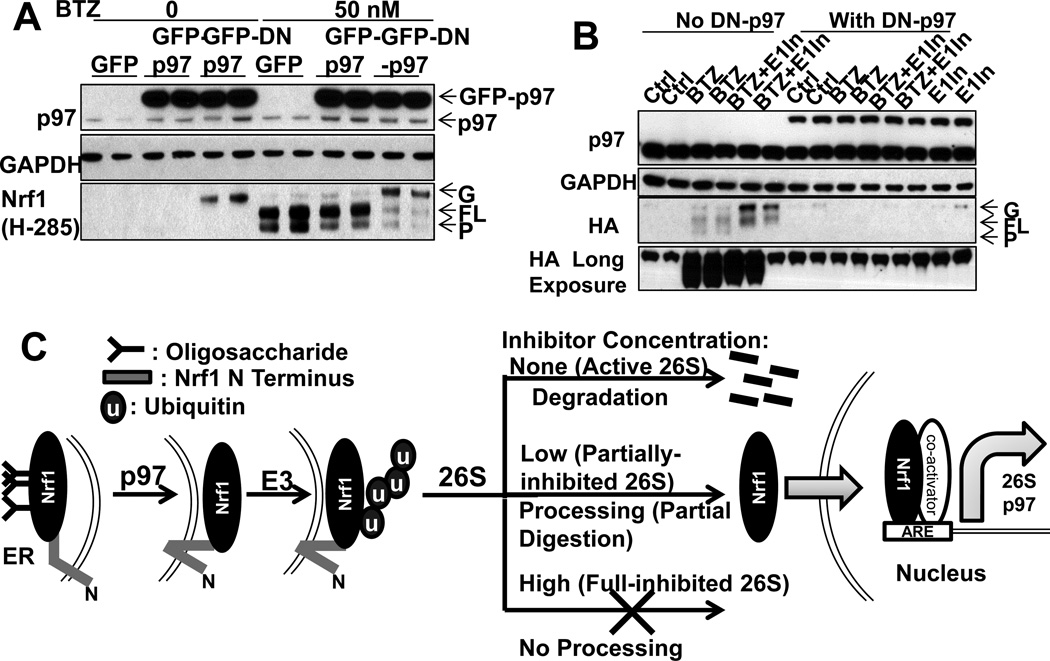
Figure 7. Nrf1 processing and the expression of 26S subunits and p97 require Nrf1 deglycosylation and p97 activity (See also Fig. S6).
(A) To test whether p97 function is essential for Nrf1 processing, HEK293A cells overexpressing GFP-p97, GFP-DN-p97, or GFP (control) were incubated with or without 50nM BTZ for 4h. GFP-DN-p97 (but not GFP-p97) reduced the level of full-length and processed Nrf1, but caused the accumulation of a 100kDa glycosylated form of Nrf1. (B) DN-p97 and Nrf1-HA were co-expressed in HEK293A cells. DN-p97 caused the sequestration of Nrf1-HA in its glycosylated form and blocked the formation of full-length and processed Nrf1 upon treatment (12h) with BTZ (20nM) or E1-In (0.5µM). (C) Model: Upon partial inhibition of proteasomes with low concentrations of inhibitors, cells induce 26S subunits and p97 via Nrf1. Although Nrf1 is normally degraded completely, partial inhibition of proteasomes by low concentrations of inhibitors favors limited degradation of the N-terminal part of Nrf1. Consequently, the processed C-terminal portion of Nrf1 is released from the ER and enters the nucleus to transcribe 26S subunits and p97. Complete inhibition of proteasomes by high concentrations of inhibitors blocks Nrf1 processing and transcription. Both Nrf1 degradation and its proteolytic processing require first deglycosylation and extraction of Nrf1 from the ER via p97 activity and then ubiquitination of Nrf1.