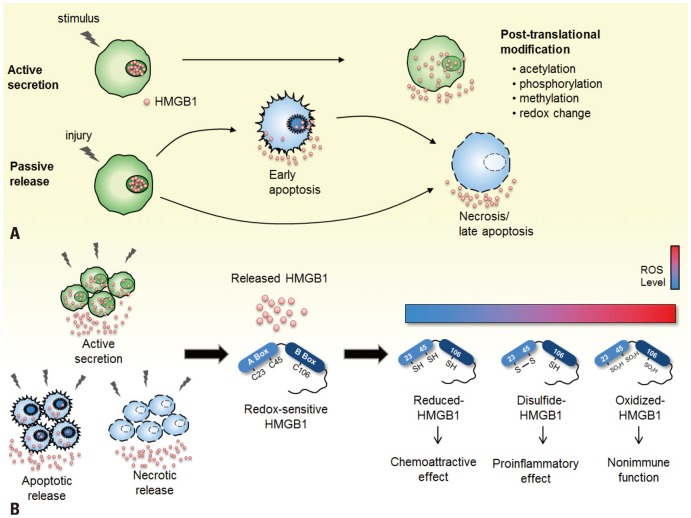
Fig. 2.
Secretion mechanism and inflammatory role of HMGB1 in redox states. (A) HMGB1 is translocated to the extracellular environment via two secretion mechanisms: active secretion by inflammatory cells or passive release by necrotic or apoptotic cells. HMGB1 in the nucleus is actively secreted when immunologically competent cells are activated by inflammatory stimulus and undergo post-translation modifications such as acetylation, phosphorylation, methylation, and redox change. Passive release of HMGB1 is mediated by necrotic or apoptotic cell death caused by injury. Released HMGB1 triggers inflammatory responses in the body. (B) The inflammatory activity of released extracellular HMGB1 is dependent upon its redox state. HMGB1 consists of three cysteine residues (C23, C45, C106) that are modified during redox change. The reduced form of HMGB1 with all thiol groups defines the chemokine activity of HMGB1, whereas the disulfide-HMGB1 with C23 and C45, forming an intermolecular disulfide bond, induces cytokine activity. The fully oxidized form of HMGB1 has no known immune function in the cells.