Abstract
Zebrafish have been increasingly used as a teaching tool to enhance the learning of many biological concepts from genetics, development, and behavior to the understanding of the local watershed. Traditionally, in both research and teaching, zebrafish work has focused on embryonic stages; however, later stages, from larval through adulthood, are increasingly being examined. Defining developmental stages based on age is a problematic way to assess maturity, because many environmental factors, such as temperature, population density, and water quality, impact growth and maturation. Fish length and characterization of key external morphological traits are considered better markers for maturation state. While a number of staging series exist for zebrafish, here we present a simplified normalization table of post-embryonic maturation well suited to both educational and research use. Specifically, we utilize fish size and four easily identified external morphological traits (pigment pattern, tail fin, anal fin, and dorsal fin morphology) to describe three larval stages, a juvenile stage, and an adult stage. These simplified maturation standards will be a useful tool for both educational and research protocols.
Introduction
Zebrafish researchers are increasingly examining developmental processes that occur during post-embryonic development.1–9 Studies of zebrafish larvae and juveniles include examination of organ maturation, physiology, gene expression, disease progression, pharmacology, toxicology, and behavior. In the last 10 years, publications discussing zebrafish larvae have increased almost sixfold, while studies on juvenile fish have increased more than threefold (PubMed search comparing 2003 and 2013). Research utilizing adult zebrafish is on the rise as well. Zebrafish are also a valuable tool for educational purposes. BioEYES, an educational program for grades 5–12, has introduced zebrafish into the classroom, engaging more than 50,000 children (bioeyes.org/ourstory.html).10 As zebrafish researchers expand their studies into larval, juvenile, and adult stages, educational programs followed suit and have begun to include later developmental stages, as shown in the study of pigment patterning developed by a set of high school students.11,12 Smaller initiatives include the use of zebrafish to teach neuroscience, development, evolution, and behavior at many academic levels.13–17
As researchers and educators progressively expand their focus onto these later developmental stages, it is crucial that additional supportive tools be developed. While several developmental staging series already exist, a simplified, elegant, yet precise, normalization table of developmental maturation would be a timely and invaluable resource. Ideally, the traits evaluated for this normalization table should be easily visible under a stereomicroscope in live fish without the need to stain or treat the fish and be easily implemented using tools commonly available in both teaching and research institutions. In addition, these stages would benefit from being linked to developmental processes. In Drosophila and Caenorhabditis elegans, clear developmental divisions occur in the form of molting. These molts define the beginning of each larval stage. Rather than having clearly defined molts, zebrafish development is a continuum of incremental changes from the single cell stage through to adulthood. Without clearly defined bounding molts for each larval stage, we should turn to other traits for which clear boundary conditions can be determined when establishing a the post-embryonic normalization table. Thus, the specific traits evaluated for a staging series should have clearly defined phenotypes that are easily identified and relevant in many different experimental conditions.
Embryonic development includes rapid morphological transformation, the endpoint of which is classically defined as the time of hatching or birth. Laboratory strains of zebrafish hatch around 3–4 days post fertilization (dpf ). The end of embryogenesis, as proposed by Kimmel et al.,18 and further utilized by Parichy et al.,19 is defined as the protruding mouth stage (∼3 dpf at 28.5°C). After the embryonic period, larval development lasts ∼6 weeks, during which the fish more than triple in length and progress through a series of morphological changes that transform the fins, pigment pattern, and overall body morphology into the juvenile configuration. In zebrafish, this occurs around 45 dpf at 28.5°C. Recent studies suggest that diet and microRNAs play a role in regulation of fish metamorphosis.20–23 Juvenile morphology closely resembles that of the adult, having lost their larval fin fold and acquired scales; however, they are not sexually mature. By ∼3 months at 28.5°C, zebrafish reach sexual maturity and are considered adults.18,19
The development of a normalization table is complicated by the many factors that can modify the rate of development. Environmental factors such as temperature, fish density, and water quality directly impact the rate of development. This is in contrast to mouse development, where the generally constant in utero environment enables development to occur at a standard rate, so embryonic age from conception can be used to define maturation. During the zebrafish embryonic period, key features of the embryo such as cell number or somite number are easily identified and are often used for defining developmental stages.18 At post-embryonic stages, age becomes less reliable as a measure of developmental maturity because early delays in maturation will be exacerbated in addition to the ongoing effects on maturation by external factors.19 While age is not an appropriate measure of maturation, fish size provides an approximation of developmental maturation. A simple strategy for defining maturation is the standard length (SL), a measure of the fish length from the tip of the snout to the base of the tail. Fish size is also influenced by genetics and other environmental factors; thus, SL in combination with assessment of the maturation of external traits is currently the most reliable measure of fish maturation.
A number of excellent normalization tables have been developed for zebrafish examining embryonic stages18,24,25 and the larvae, juvenile, and adult forms.19 For example, the Parichy et al.19 normalization table of development is extremely thorough and examines many distinct and readily defined externally visible traits. It also accounts for shrinkage due to fixation, the effect of temperature, and it standardizes slight discrepancies between fish size and maturation of defined traits. As a consequence, the Parichy normal table is very complex. Such refined developmental staging is not always needed. Here, we propose a simplified table focusing on five key developmentally evident traits: SL, pigmentation, tail fin, anal fin, and dorsal fin. Together, these traits are used to define hallmark phenotypes that mark the beginning of three distinct larval periods, as well as the juvenile and the adult stages. This simple normalization table highlights significant developmental events and can easily be implemented in educational and research settings.
Materials and Methods
Zebrafish
Wildtype zebrafish (AB) were grown in a facility at 28°C±1°C, with water and air temperature readings collected daily. Individual mating pairs were crossed in mating tanks (Aquatic Eco-systems, Inc.), and the resulting eggs were screened for overall health and development from embryo collection to 4 dpf. After 5 days in a laboratory incubator at 28.5°C, fish were separated into tanks at a density of 10 fish per 2 L tank. At 20 dpf, the fish were checked for health and sorted to maintain a tank density of 10–12 fish per 2 L tank in a flow through the water system (Aquatic Innovation LLC). Three different sibling clutches were raised over the course of 2 years to account for the effect of possible variations in growth conditions on the observed traits. A total of 175 fish were examined. Fish were collected at larva through adult stages: 15, 30, 45, 60, 90, and 180 dpf. They were fixed in 4% paraformaldehyde (PFA) overnight at 4°C. Fish were rinsed three times in phosphate-buffered saline and photographed using a stereomicroscope (SteREO Discovery V12; Zeiss) and camera (AxioCam MRc; Zeiss) with its associated image acquisition software (AxioVision; Zeiss). In addition, the standard length (SL), measured from snout to the base of the tail, was measured directly from the fixed fish using digital calipers or directly from scale calibrated images with ImageJ (NIH). Measured SL was adjusted for fixation shrinkage by adding 0.29 to each SL value as suggested by Parichy et al.19
Two pigment mutations were also examined: cx41.8t1 mutants26 have leopard spots, and slc45a2b4 fish are albino and lack the ability to make melanin.27 All measurements and imaging of the pigment mutations were conducted on live fish, anesthetized in MS222. All additional analysis was conducted as for the wildtype fish.
Maturation standards
We characterized the phenotype of four external body traits and created five distinct phenotype classifications for each physical trait; pigmentation phenotype (PP1, PP2, PP3, PP4, and PP5) and morphology of the tail fin phenotype (TP1, TP2, TP3, TP4, and TP5), anal fin phenotype (AP1, AP2, AP3, AP4, and AP5), and dorsal fin phenotype (DP1, DP2, DP3, DP4, and DP5). The defining hallmarks of the start of each classification are summarized in Table 1 and Figure 6. To validate our classification system, we used it to stage the images of fish presented in the Parichy et al. post-embryonic staging series paper.19 We found that pigment was the least reliable trait for comparison, as the ABwp and ABwp crossed with wikwp strains used in the Parichy paper acquire the pigment phenotypes used in our maturation standard at slightly larger SL than our AB fish. Examination of the pigment mutants also demonstrates the difficulty of using pigment as a marker for the developmental stage.
Table 1.
Defining Phenotypic Boundaries
| Pigment pattern | Tail fin | Anal fin | Dorsal fin | |
|---|---|---|---|---|
| Phenotype 1 | Single spotted line of melanophores (black pigment) along the lateral line. | No fin rays. Tail fin is rounded in shape and composed of the fin fold only. | No fin rays. Anal fin fold is clearly visible. | No fin rays. Dorsal fin fold is clearly visible. |
| Phenotype 2 | Dispersal of melanophores above the lateral line. Condensation of melanophores over the swim bladder and cranial region. | Tail fin rays emerge. Tail fin initially rounded, then develops two slightly pointy tips. | Anal fin rays emerge. Fin has rounded form. | Dorsal fin rays emerge. Fin has rounded form. |
| Phenotype 3 | Two distinct melanophore stripes on either side of the lateral line, extending to the tail. | Tail fin rays have a single tip elongated toward the fin margin and that widens at distal end (no fork). | Anal fin rays have a single tip elongated toward the fin margin and that widens at distal end (no fork). | Dorsal fin rays have a single tip elongated towards the fin margin and that widens at distal end (no fork). |
| Phenotype 4 | Three distinct stripes of melanophores running laterally along the fish length. The pigment density in stripe 1 and 2 has increased. | Tail fin rays have begun to fork and extend to the fin margin. Sixteen to 17 tail fin rays are clearly visible. | Anal fin rays have reached the margin of the fin and begun to fork. Twelve anal rays are clearly visible. | Dorsal fin rays have reached the margin of the fin and begun to fork. Five to seven dorsal rays are clearly visible. |
| Phenotype 5 | Four distinct stripes of melanophores with the fourth emerging dorsally. Stripes 1–3 have dense melanophore distribution. | Tail fin rays begin to fork a second time. Eighteen to 24 tail fin rays. | Anal fin rays begin to fork a second time. Twelve to 14 anal fin rays. | Dorsal fin rays begin to fork a second time. Eight to 10 dorsal fin rays. |
The fundamental defining feature of each phenotype is identified. In each case, the hallmark defining the transition to the next phenotype is indicated. It should be noted that while the pigment pattern changes progressively, the fin phenotypes are defined by clear morphological events.
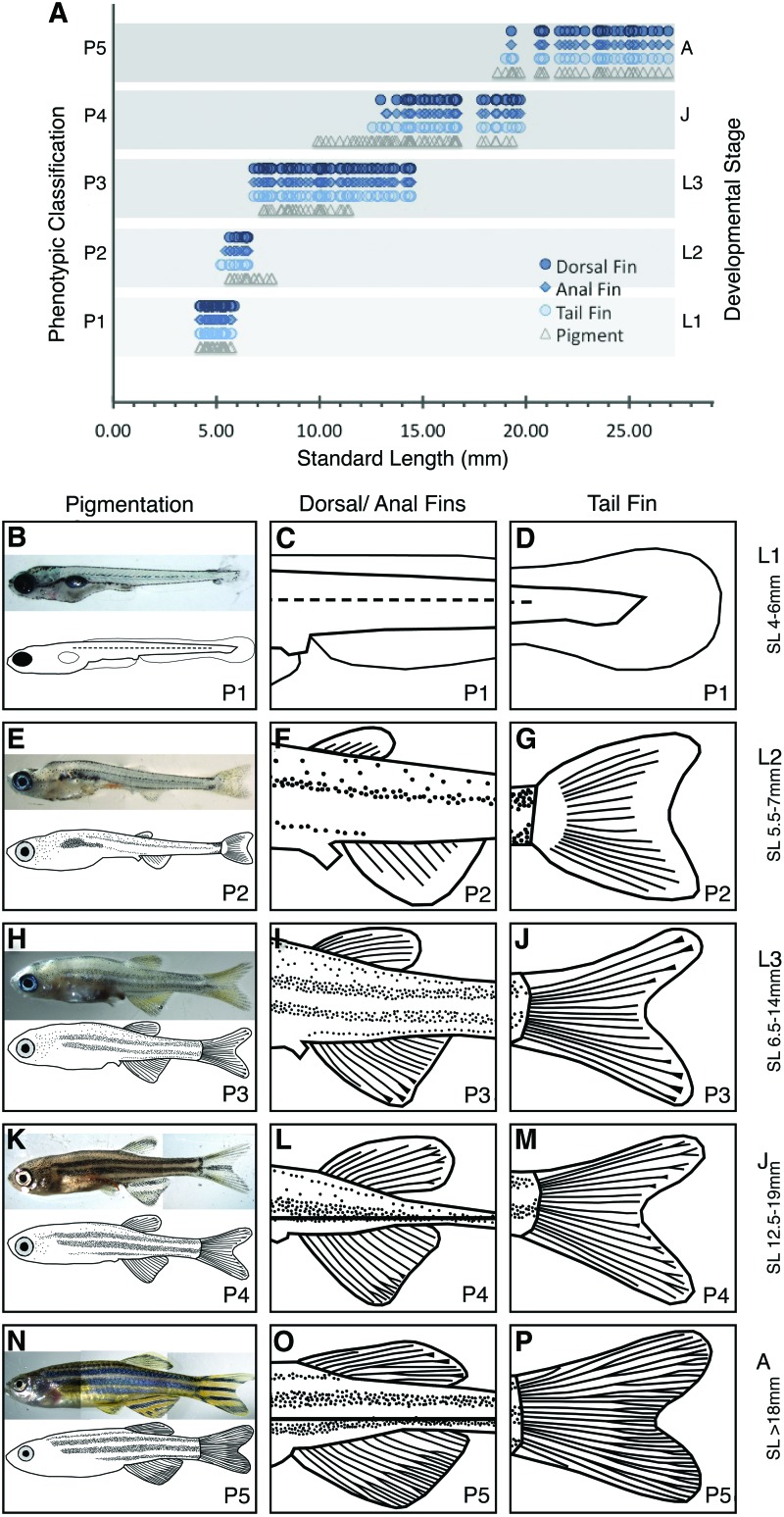
FIG. 6.
Schematics of each larval stage (L1, L2, and L3), juvenile (J) and adult (A) stages. (A) The graph shows the distribution of all the traits (pigment, tail, anal fin, and dorsal fin morphology) concurrently compared with SL. The three fin phenotypes are distributed over defined size ranges, while the pigment phenotypes demonstrates a wider range in size. Thus, we focus on the fin phenotypes to define the developmental stage. (B) L1 archetypical zebrafish and schematic with phenotypes of P1 for all traits. (C) Close-up schematic of dorsal and anal fins for P1 phenotypes of each trait. (D) Close-up schematic of P1 tail. (E) L2 archetypical zebrafish and schematic with phenotypes of P2 for all traits. (F) Close-up schematic of dorsal and anal fins for P2 phenotypes of each trait. (G) Close-up schematic of P2 tail. (H) L3 archetypical zebrafish and schematic with phenotypes of P3 for all traits. (I) Close-up schematic of dorsal and anal fins for P3 phenotypes of each trait. (J) Close-up schematic of P3 tail. (K) Juvenile archetypical zebrafish and schematic with phenotypes of P4 for all traits. (L) Close-up schematic of dorsal and anal fins for P4 phenotypes of each trait. (M) Close-up schematic of P4 tail. (N) Adult archetypical zebrafish and schematic with phenotypes of P5 for all traits. (O) Close-up schematic of dorsal and anal fins for P5 phenotypes of each trait. (P) Close-up schematic of P5 tail. Color images available online at www.liebertpub.com/zeb
Every fish was quantified for each physical trait from the photographs, and data were recorded in Excel 2004 (Microsoft). All data analysis and graphs were generated in Excel 2004 (Microsoft), and all images were processed in Photoshop CS5 (Adobe Systems, Inc.). Schematic images and final figures were made using InDesign CS5 (Adobe Systems, Inc.). The phenotypic classifications (1–5) for each trait were plotted against the SL (Figs. 2F–5F), and all the physical traits were plotted together against the SL (Fig. 6A).
FIG. 2.
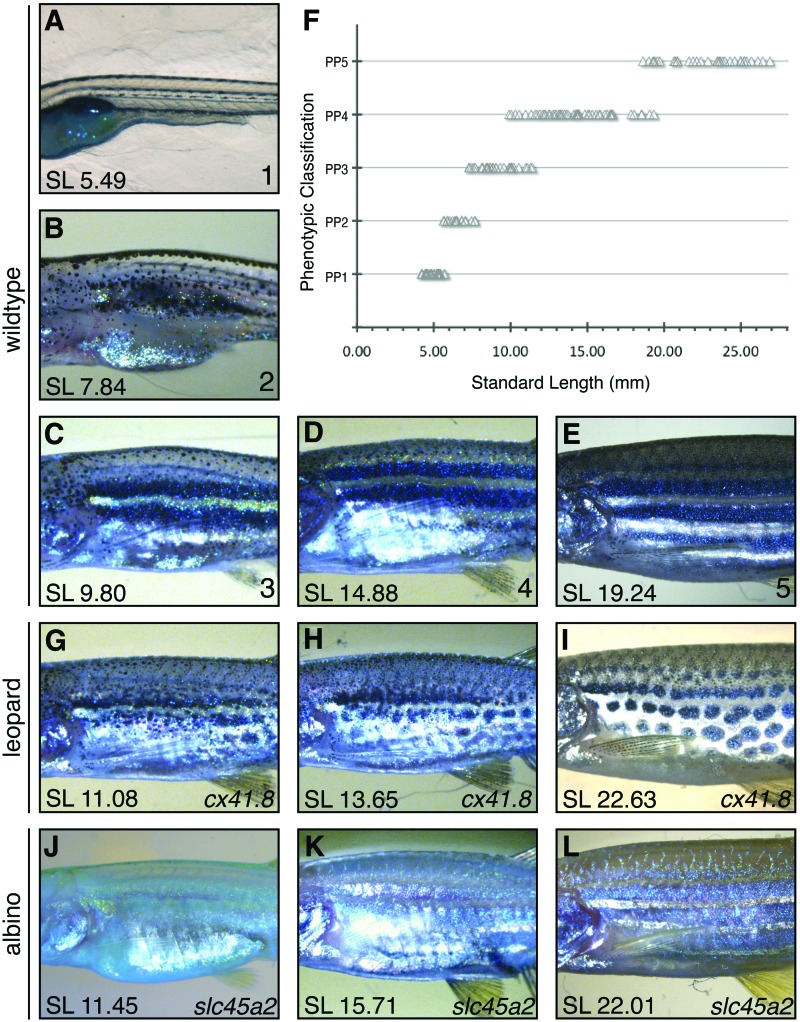
Pigment in the zebrafish varies distinctly throughout development, beginning with fish that are nearly optically clear, through adult fish with distinct patterns of yellow and black pigment. (A) Pigment phenotype 1 (B) pigment phenotype 2 (C) pigment phenotype 3 (D) pigment phenotype 4 (E) pigment phenotype 5. (F) The distribution of pigment phenotypes 1–5 as the larval fish grows to adulthood. (G–I) Leopard pigmented fish (cx41.8t1) size matched to appropriate wildtype fish of PP3, PP4, and PP5. (J–L) Albino, slc45a2b4 mutant, fish size matched with PP3, PP4, and PP5 wildtype fish. Color images available online at www.liebertpub.com/zeb
FIG. 3.
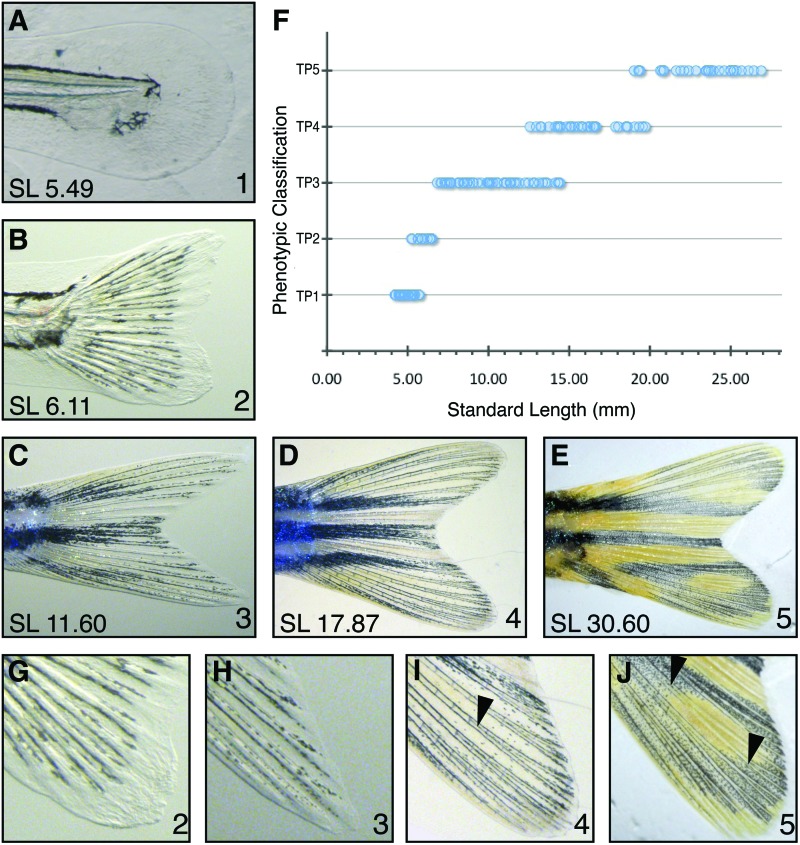
The tail fin morphology changes from a simple fin fold to a rayed fin. (A) Tail fin phenotype 1 (B) tail fin phenotype 2 (C) tail fin phenotype 3 (D) tail fin phenotype 4 (E) tail fin phenotype 5. (F) The distribution of tail fin phenotypes 1–5 by SL. (G) Close up of tail fin phenotype 2. (H) Close up of tail fin phenotype 3. (I) Close up of tail fin phenotype 4. (J) Close up of tail fin phenotype 5. Arrowheads indicate forking points of fin rays. Color images available online at www.liebertpub.com/zeb
FIG. 4.
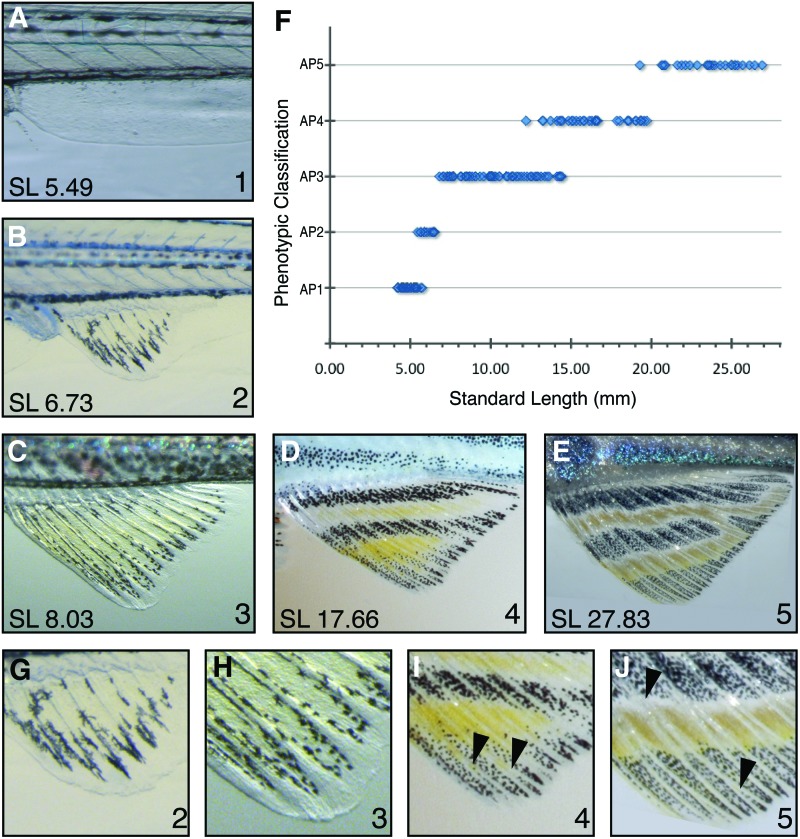
The anal fin morphology changes from a simple fin fold to a rayed fin. (A) Anal fin phenotype 1 (B) anal fin phenotype 2 (C) anal fin phenotype 3 (D) anal fin phenotype 4 (E) anal fin phenotype 5. (F) The distribution of anal fin phenotypes 1–5 by SL as the fish grows to adulthood. (G) Close up of anal fin phenotype 2. (H) Close up of anal fin phenotype 3. (I) Close up of anal fin phenotype 4. (J) Close up of anal fin phenotype 5. Arrowheads indicate forking points of fin rays. Color images available online at www.liebertpub.com/zeb
FIG. 5.
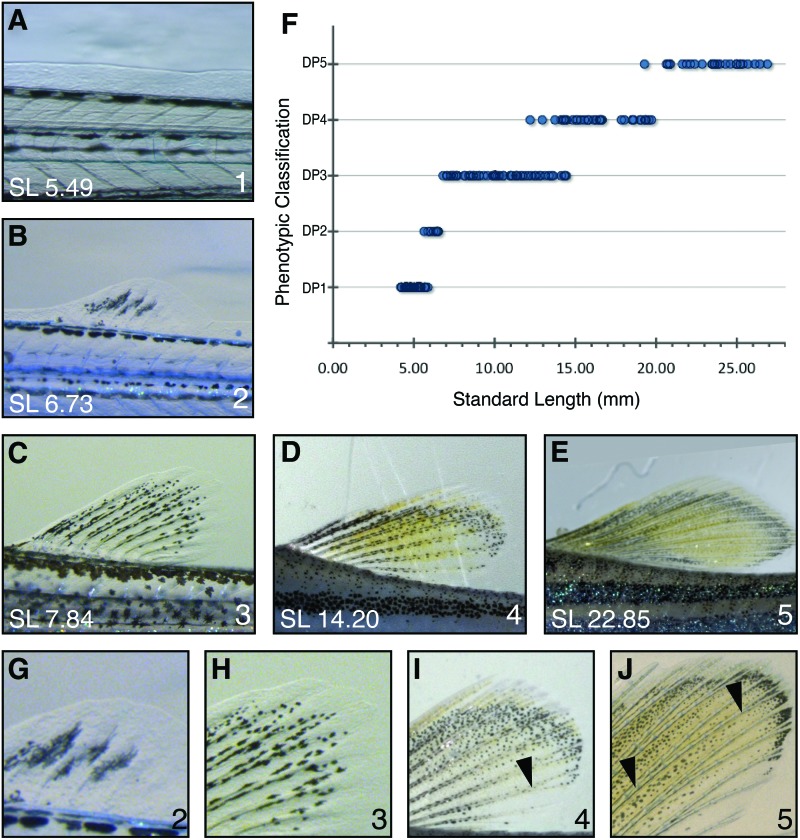
The dorsal fin morphology changes from a simple fin fold to a pigmented rayed fin. (A) Dorsal fin phenotype 1 (B) dorsal fin phenotype 2 (C) dorsal fin phenotype 3 (D) dorsal fin phenotype 4 (E) dorsal fin phenotype 5. (F) The distribution of dorsal fin phenotype 1–5 by SL. (G) Close up of dorsal fin phenotype 2. (H) Close up of dorsal fin phenotype 3. (I) Close up of dorsal fin phenotype 4. (J) Close up of dorsal fin phenotype 5. Arrowheads indicate forking points of fin rays. Color images available online at www.liebertpub.com/zeb
Results
Maturation and growth rate
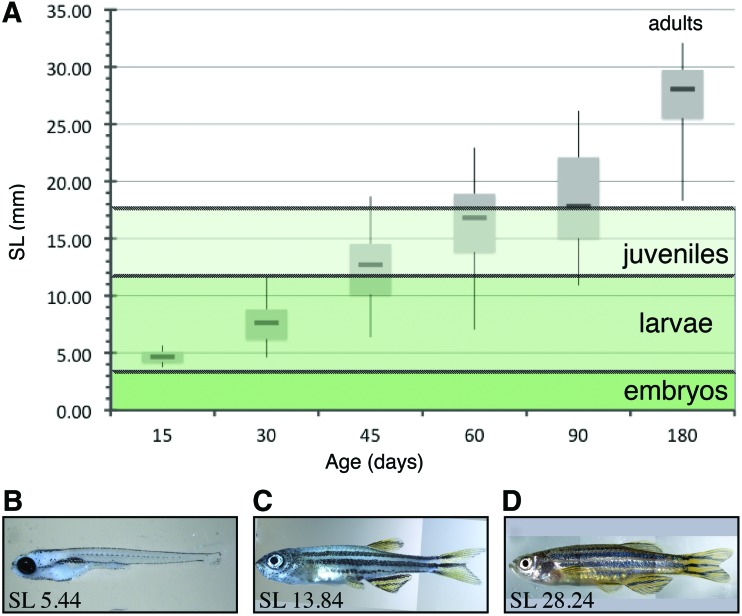
During zebrafish development, the rate of zebrafish growth is influenced by many factors: water quality, temperature, genetics, food quality and availability, and population density. Even zebrafish grown in standardized conditions show a range of sizes and maturation states as they age (Fig. 1A). Examination of the growth and maturation of three distinct sibling sets of wildtype (AB) zebrafish over the course of 2 years reveals a range of body sizes at any given age (Fig. 1). Zebrafish are generally considered juveniles at 45 dpf and adults at 90 dpf. However, the size distribution of an age matched cohort suggests that while the majority of fish have reached the size defining the juvenile stage (12 mm SL) at 45 days and adult stage (18 mm SL) at 90 days,18,19,24,25 others of the same age have yet to reach the critical size (Fig. 1A). This suggests that size alone is insufficient to define the maturation state of the fish. Examining the external maturation state of the fish at each age (Fig. 1B–D) also reveals a range of phenotypes, further supporting the idea that zebrafish age alone is not a satisfactory method for defining maturation state. In contrast, zebrafish length, measured from the snout to the base of the tail (SL), in combination with easily observed morphological traits, such as pigment pattern and fin morphology, are more reliable markers for the zebrafish maturation stage.19
FIG. 1.
Zebrafish grow continuously over time and at variable rates such that at any given age, they range considerably in size. (A) Box plot comparing standard length (SL) with age. The central bar is the median while the box represents the inner quadrants, the area where most of the data is condensed, and the vertical extensions show the extreme upper and lower extent of the fish sizes. (B) Representative 15 days post fertilization (dpf ) zebrafish exhibiting larval morphology. (C) Representative zebrafish of transitional phenotype seen in juveniles. (D) Representative 180 dpf fish of clear adult phenotype. Color images available online at www.liebertpub.com/zeb
In order to define a simplified normalization table for zebrafish maturation, we measured each fish for its SL and examined the fish for four externally evident traits: pigmentation pattern, tail fin morphology, anal fin morphology, and dorsal fin morphology. The four traits were chosen, because they are easily visualized with standard laboratory equipment such as the stereomicroscope and camera. For each trait, we identified five distinct phenotypic classifications with clear boundaries for each. While pigment pattern demonstrates a slow transition throughout development from embryonic stages to adulthood, we identified five distinct fin ray phenotypes that we suggest mark clear developmental stages. In combination, the four phenotypic traits along with the SL can be used to define five distinct maturation states: three larval stages (L1, L2 and L3), a juvenile stage (J), and the adult stage (A).
Pigment pattern development
Pigment patterns are an easily observable morphological trait whose pattern is linked to zebrafish maturation.28 After careful examination, we defined five distinct classifications of pigment patterns observable during maturation of the fish. Zebrafish classified as pigment phenotype 1 (PP1) have a spotted line of melanophores (black pigment) along the lateral line (Fig. 2A) and have a SL of 4.0–6.0 mm. Further development results in pigment phenotype 2 (PP2), in which a condensation of melanophores has formed over the swim bladder. The melanophores also begin dispersing above the lateral line, in PP2 (Fig. 2B), and fish range in size from 5.5 to 7.5 mm SL. By the time the larvae approach 8.0 mm SL, most fish have developed two clear melanophore stripes along their flank, both dorsal and ventral to the lateral line, defining pigment phenotype 3 (PP3; Fig. 2C). PP3 classified fish have an SL of 7.5–11.5 mm SL. Since the transition from larvae to juvenile stages occurs at ∼12.0 mm, PP3 marks the last of the larval stages. A third distinct lateral stripe of melanophores develops ventrally to the previous two stripes (Fig. 2D) defining pigment phenotype 4 (PP4). This phenotype marks the transition to the juvenile stage, and PP4 zebrafish range in size from 10.0 to 19.5 mm SL. By adulthood, a fourth stripe of melanophores has emerged dorsal to the three stripes identified in PP4, thus defining pigment phenotype 5 (PP5; Fig. 2E). PP5 fish have an adult pigment pattern and all have an SL of 18.5 mm or larger, consistent with the previously defined adult fish size.
In order to determine whether the identified pigment patterns correlate with growth and maturation, we plotted the phenotypic classification against fish size (Fig. 2F) and found that the pigmentation phenotypes group within particular size ranges and overlap at the edges of the size range with the adjacent phenotypes. These five phenotypic classifications for pigment patterns clearly progress as the fish increases in SL; however, at some lengths, there is significant overlap in the phenotypic classification. Specifically, an individual zebrafish with an SL of 11.0 mm may be defined by its pigment pattern as either PP3 or PP4. Similarly, a fish with an SL of 19.0 mm may be classified as either PP4 or PP5 depending on the observed phenotype (Fig. 2F). For a summary of the key features that define the beginning of each pigment phenotype, see Table 1.
An additional complicating factor when using pigment as a marker for maturation is the presence of pigment mutations or experimental procedures that disrupt pigment pattern. A number of pigment mutations are used in zebrafish research and education, including the classic mutation, cx41.8t1, which produces leopard spots,26 and slc45a2b4, the albino mutant (Fig. 2G–L). At early developmental stages, cx41.8t1 mutants are hard to distinguish from their wildtype counterparts (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/zeb). However as the cx41.8t1 fish grow, disruptions in the pigment pattern become evident (Fig. 2G). Soon clusters of melanophores form, resulting in the leopard spot pattern rather than the typical stripes (Fig. 2H, I). These differences in pigment patterning make it difficult to place cx41.8t1 mutations within our pigment phenotype classifications. This problem is exacerbated in the albino mutant, slc45a2b4,27 which lacks the ability to produce melanin and thus lacks all the dark pigment (Fig. 2J–L and Supplementary Fig. S1). The slc45a2b4 fish still produce xanthophores (yellow) and iridophores (reflective), but all the dark pigmented stripes are missing. Therefore, we should look beyond pigment to generate a broadly applicable, simplified maturation standard.
Tail fin development
After close examination of the tail fin throughout development, we identified five morphologically distinct defining characteristics that may mark key developmental transitions. In contrast to the progressive changes in pigment pattern development, tail fin development has hallmarks that clearly delineate boundary conditions between each phenotypic classification. Fin development and maturation transform the embryonic zebrafish into an archetypically shaped fish. The tail in the zebrafish begins development as a rounded fin fold with no defined fin rays, as is seen in larvae with tail fin phenotype 1 (TP1; Fig. 3A). TP1 fish have an SL of 4.0–6.0 mm (Fig. 3F). As the fin rays emerge, the shape of the tail fin transitions from blunt to forked, a process beginning with two slight points at the ventral and dorsal regions of the tail. The presence of condensed fin rays marks the beginning of tail phenotype 2 (TP2). Zebrafish, with TP2 classification, range in size from 5.5 to 7.0 mm SL (Fig. 3B, F and G). Tail phenotype 3 (TP3) larvae exhibit tail fin rays that reach the margin of the tail fin, and these rays begin to thicken at their distal tips (SL 6.5–14.0 mm; Fig. 3C, F and H). The thickened fin ray tips begin to fork as the tail fin grows and the fin rays lengthen (Fig. 3D, I arrowhead). This initial fin ray forking is the indicator for the beginning of tail phenotype 4 (TP4). Fish exhibiting TP4 are 12.5–19.0 mm SL (Fig. 3F), supporting the model that entry into TP4 corresponds to the transition to the juvenile stage. As the fish grow to adulthood, the tail fin rays will fork once more as they continue to grow. This second forking event marks the initiation of tail fin phenotype 5 (TP5; Fig. 3E, J arrowheads) and is observed in fish with an SL >19.0 mm (Fig. 3F), suggesting that TF5 marks entry into the adult stage. For a summary of the key features that define the beginning of each TP phenotype, see Table 1.
The presence of pigment in wildtype tail fins helps visualize the fin rays as the melanophores outline the fin rays (Fig. 3). The absence of tail fin stripes is also a feature of TP1 and TP2; however, the tail fin phenotype classifications are not dependent on pigment pattern. The same patterns of fin ray maturation are observed in both cx41.8t1 and slc45a2b4 mutants throughout development (Supplementary Fig. S2). Together, these data suggest that, more generally, fin ray morphology may serve as a useful phenotype for defining developmental stages throughout post-embryonic development.
Tail fin development occurs in a stepwise fashion, likely reflecting key developmentally regulated events, with each step revealing a distinct characteristic that defines the starting point for the tail fin phenotypic classifications (Table 1). These classifications align nicely with fish size (Fig. 3F) such that each phenotype forms a clear size-related group. There is some overlap in the size range of each phenotypic class with the adjacent one, but each classification clusters with very few outliers. TP1–3 represent larval phenotypes, while TP4 marks entry into the juvenile stage and TP5 marks entry into adulthood.
Anal fin development
As with tail fin development, five clear phenotypic classifications of anal fin development can be defined. Anal fin development begins with a clearly visible fin fold with little or no internally defined morphology. We define this stage as anal fin phenotype 1 (AP1, Fig. 4A); these fish have an SL of 4.0–6.0 mm (Fig. 4F). The anal fin fold becomes a distinct rounded anal fin, within which fin rays begin to condense (Fig. 4B, G). This occurs when the fish are between 5.5 and 7.0 mm SL (Fig. 4F). Fin ray condensation defines the beginning of anal fin phenotype 2 (AP2; Fig. 4B, G). As the fish grows, the anal fin lengthens and the distal tips of the fin rays thicken in preparation for forking. Fin ray thickening near the fin margin defines anal fin phenotype 3 (AP3; Fig. 4C, H). Fish characterized as being at AP3 are 6.5–14.0 mm SL (Fig. 4F). By this point in development, 12 fin rays are evident. Anal fin phenotype 4 (AP4) is defined by the newly formed forks within the thickened fin rays (Fig. 4D, I arrows). Similar to TP4, AP4 marks the transition to the juvenile form, as fish classified as AP4 range in size from 13.0 to 19.5 mm SL (Fig. 4F). A second anal fin ray bifurcation (Fig. 4J arrowheads), similar to that observed in the TP5, occurs in the anal fin and defines anal fin phenotype 5 (AP5; Fig. 4E, J). The adult phenotype is represented by AP5 and is observed in the largest fish with an SL of 19.0 mm or greater. A summary of the features that define the start of each anal fin classification can be found in Table 1.
Anal fin development can also be characterized by pigment pattern maturation. When characterizing the pigmentation in our previously defined AP classifications, a pattern of pigment development emerges. AP1 and AP2 fish lack stripes, as they arise later in development; however, melanophores can be seen in the AP2 fins. AP3 fins have an increased number of melanophores, and the xanthophores begin to emerge. By AP4, the fish have anal fins with a melanophore stripe at the fin base and melanophores also condensed at the tip of the fin (Fig. 4D, I). Adult zebrafish have anal fins with two or more distinct melanophore stripes alternating with xanthophores representing AP5 (Fig. 4E, J). These pigment patterns enhance our analysis of each anal fin classification. To demonstrate the functionality of utilizing fin ray development rather than depending on pigment pattern to define our anal fin classification, we observed fin ray maturation in both cx41.8t1 and slc45a2b4 mutants throughout development and found the same pattern of development seen in wildtype fish (Supplementary Fig. S3). The fin rays in these pigment mutant fish and all AP classifications are easily identifiable. Thus, the presence of pigment provides an additional opportunity for evaluating the phenotypic classification, but it is not required.
As seen with body pigment patterning and tail fin maturation, zebrafish progress through these defined anal fin phenotypic classifications. Clear size-dependent phenotypes are observed (Fig. 4F), and each classification has clearly defined boundaries. Again, three larval classifications are defined (AP1–3) followed by the juvenile and adult classifications, AP4 and AP5, respectively.
Dorsal fin development
Early development of the dorsal fin begins with the formation of a fin fold (Fig. 5A); we define this stage as dorsal fin phenotype 1 (DP1). DP1 includes fish that range from 4.0 to 6.0 mm SL (Fig. 5F). Cells within the fin fold begin to condense to form the fin rays, while the fin takes on a rounded form. When fin rays do not yet reach the fin margin, fish are classified as having dorsal fin phenotype 2 (DP2; Fig. 5B, G). These fish range from 5.5 to 7.0 mm SL (Fig. 5F). Dorsal fin phenotype 3 (DP3) is characterized by dorsal fins with elongated fin rays that reach the fin margin and though these fin rays thicken at the distal tips, there are no forked fin rays present (Fig. 5C, H). DP3 can be found in fish 6.5–14.5 mm SL (Fig. 5F). DP3 marks the end of the larval period. Fish larger than 12.5 mm are considered juveniles and show single forks at the tip of the some of the fin rays. This dorsal fin phenotype is defined as dorsal fin phenotype 4 (DP4; Fig. 5D, I) and is found in fish between 12.5 and 19.5 mm SL. The dorsal fin rays will divide again, generating a second fork and defining the start of dorsal fin phenotype 5 (DP5; Fig. 5E, J). This is the predominant phenotype in fish with an SL of more than 19.0 mm and is thus defined as the adult form. A summary of the features that define the start of each dorsal fin classification can be found in Table 1.
Pigment changes are also evident in the developing dorsal fin. Initially, in DP1 fins, there is little or no pigment in the fins (Fig. 5A). DP2 fins have melanophores that cluster around the developing fin rays (Fig. 5B, G). As the fin grows, so do the fin rays and the melanophores adjacent to the rays begin to disperse along the length of the rays. Xanthophores begin to emerge at DP3 (Fig. 5C, H). By DP4, dorsal fins have developed xanthophores throughout the fin (Fig. 5D, I). Adult dorsal fins (DP5) have a distinct melanophore stripe along the dorsal ridge and along the rays of the fin in addition to melanophores distributed along and between the fin ray margins (Fig. 5E, J). While the presence of defined pigment patterns aids in identifying a developmental stage, fin ray morphology alone is sufficient for classification; we observed fin ray maturation in both cx41.8t1 and slc45a2b4 mutants throughout development and were able to classify these mutants in the same way as wildtypes (Supplementary Fig. S4).
Defining the simplified normalization table of development
Combining the analysis of the standard length and all four phenotypic trait classifications reveals five distinct developmental stages (Fig. 6A). It should be noted that it is possible to find a range of phenotypic classifications for the traits in an individual fish. In this case, we define the developmental stage based on the most frequent classification identified. The phenotypic classifications of all traits group together within a defined but overlapping series. Three of these groups occurring within the larval size range, one within the juvenile size range and the largest classification representing adults. We define the first larval size group as L1, and this stage includes fish in which the majority of the phenotype classifications are P1 (Fig. 6B–D). The second larval stage (L2) defines the developmental stage for fish with the majority of traits classified as P2 (Fig. 6E–G). It should be noted that during L2, the fin traits group very tightly while the pigment classification for PP2 is shifted to a slightly larger size in comparison to the fin classifications. The third and last larval stage (L3) is defined by the P3 classifications for the majority of the traits (Fig. 6H–J). The pigment phenotype transitions between P3 and P4 earlier than the fin phenotypes, making the pigment pattern a less valuable tool for stage assessment in fish ranging from 10 to 13 mm. Previous work has defined the juvenile stage as beginning at ∼12 mm SL. Since the pigment phenotypes are less valuable at this stage, the P4 fin classifications (Fig. 6K–M), which start at ∼12.0 mm SL, define the juvenile (J) stage. Similarly, adult zebrafish are generally longer than 18 mm SL. P5 classifications, including pigment (Fig. 6N–P), generally define larger fish beginning at 18.0 mm SL (Fig. 6A). Fish with the majority of the classifications that are P5 are considered adults.
The phenotypic classifications for each of the evaluated traits demonstrate clear size dependence such that the progressive classifications, P1–5, represent increasingly larger fish. Individual fish transition between phenotypic classifications at slightly different sizes, so there is a region of phenotypic overlap at the margins of each classification. It is likely that this variation in development is due to small variations in the relationship between size and factors that regulate maturation. The presence of such a small region of phenotypic overlap is an indication of the strength of the boundary conditions imposed on each classification. Similarly, the obvious grouping of phenotypic classifications by size strengthens the value of using the proposed classification system as a method to stage zebrafish development. It is also obvious that size alone is not sufficient to predict phenotypic class evaluation and at least one other trait is necessary. While each trait on its own demonstrates a clear size relationship, we combined all the trait data into a unified table that easily and reliably defines developmental stages (Table 1).
Using the simplified normalization table of development
When using the simplified normalization series, zebrafish larvae can be observed directly, imaged live or fixed. When calculating the standard length (SL), we should recall that the fish will shrink in some fixatives such as 4% PFA so a size correction may be required.19 We suggest measuring the SL as a starting point for determining the maturation stage. Phenotypic classification of the fins should directly follow SL measurement, as these traits are more robust measures of maturation state. Lastly, we should evaluate the pigment phenotype while keeping in mind that this trait is the least accurate measure of the maturation stage. We have found that generally the fin phenotypic classifications for any given fish correlate well with each other and with the SL. Once each trait is evaluated and the SL is determined, the developmental stage should be defined as the stage most frequently identified using these criteria. For example, if a fish is 6.2 mm, and all the fin phenotype classifications fall within P2 but the pigment phenotype is a P3, this fish should be defined as an L2 larvae.
Discussion
Zebrafish are increasingly being used to study processes beyond the embryonic stages in larval, juvenile, and adult fish. There is a detailed discussion of post-embryonic zebrafish maturation presented by Parichy et al.19 This article explores many morphological traits characterizing the differences between larval, juvenile, and adult stages and is complex. Obviously, for some research and for educational uses, there could be advantages to using a simplified maturation table to determine the developmental stage of larvae, juveniles, and adult zebrafish. This study defines a comprehensive staging table using SL and four easily observable external traits to divide larval development into three defined larval stages (L1, L2, and L3), juvenile (J) and adult (A) life stages. It is important to note that all the stages presented here are in fish 15 days or older and thus, would be considered vertebrate animals; students should check the rules for use of vertebrate animals at educational facilities and for use in science fair projects.
Determining the SL of a zebrafish is a simple way to determine an approximate developmental stage; however, there may be experimental or growth conditions that make this measure insufficient, and additional traits of maturation are useful for defining maturation state. These traits should be easily observable without invasive methods, such that analysis of live fish is possible without stressing or damaging the zebrafish. We examined four traits: pigment pattern, tail, anal, and dorsal fin maturation. Each trait was characterized using carefully defined criteria (Table 1) at multiple times during development and divided into five distinct phenotypic classifications (P1–5; Figs. 2–5). Each trait on its own can be useful in determining maturation state, with pigment being the most variable, but when combined, a definitive pattern emerges. During larval development, as defined by SL of 3.5–12 mm, three distinct phenotypic groups are observed. Each of these phenotypic groups represents a larval stage. As SL increases, two more groups defining juvenile and adult stages are observed (Fig. 6 and Table 1).
During the larval periods, most individual zebrafish demonstrate all the phenotype classifications that correspond to their L1–3 larval stage. For example, an individual fish with phenotype classifications of TP1, AP1, and DP1 will be defined as L1. The tight correlation of the observed fin traits suggests that they develop in tandem. One benefit of this observation is that in many cases it will be sufficient to define only one of the fin phenotypic classifications, along with SL to determine the developmental stage. This also means that this normalization table can be used to classify conditions in which one of the other traits is modified by mutation or environmental conditions, such as with pigment mutations. Pigment maturation is the least reliable means of defining maturation state, particularly during the transition from the larval to juvenile stages. For instance, the pigment pattern transition from PP3 to PP4 occurs earlier than the transformation of the P3 to P4 fin phenotypes.
We went on to compare our pigment phenotypes with those published in the Parichy et al.19 post-embryonic staging series and found that our strain of wildtype AB fish acquire more mature pigment patterns at a slightly smaller SL than the ABwp and ABwp crossed with wikwp strains examined in that paper. These findings suggest that pigment pattern may have limited value in defining developmental stages. In addition, the Parichy paper does not discuss the presence of forks during fin formation, so these phenotypes are difficult to compare. However, the initial formation of the fin rays observed in Parichy et al.19 corresponds to when we observe their initial formation.
The phenotype classifications for tail, anal, and dorsal fins cluster very tightly by size, suggesting that our classification system may reveal a common regulatory mechanism that coordinates the timing of key fin developmental events such as fin ray forking. There is some variance in the size ranges of the pigment phenotype as compared with the fin phenotypic classifications. This may reflect the progressive maturation of pigment patterning or may indicate that there is greater flexibility in the timing of pigment pattern development.
This normalization table divides the larval period into three distinct stages based on four easily observed external traits. There are many possible applications of the simplified staging series, which has particular value for classifying larval stages. Larval development starts at ∼3 days in development and ends around 45 dpf when fish are grown under optimal conditions. It is widely known that hormones play a role in directing morphogenesis in many animals, from amphibians to insects. In insects, many larval stages are defined by hormone-induced molts. Each molt marks the end of one larval period and the beginning of the next. Zebrafish do not have such defined morphological transformations, yet it is likely that hormones, such as the thyroid hormones T3 and T4, play roles in directing metamorphic transformations in zebrafish. Thyroid hormone levels fluctuate during development with T3 levels peaking at 10 dpf and T4 levels peaking at 21 dpf.29 The T3 hormone peak may regulate the transition from embryo to larva, while the T4 peak occurs when the fish are predicted to be ∼6.5 mm SL, at the transition between L1 and L2. In addition, inhibition of thyroid hormone in zebrafish prevents the transformation from the larval to the juvenile form.30 Hormone fluctuations likely drive the progression of key morphological traits during development.
The simplicity of this staging series lends itself well to both research and educational uses. Currently, many educational tools focus on embryonic development such as BioEYES.10 However, researchers are increasingly examining aspects of later growth and maturation and so, educational programs are also increasingly examining these later stages. One obvious example of the use of larvae in the classroom is a student-driven educational module developed at Sidwell Friends School in Washington D.C.12, where students examine pigment pattern formation in normal and mutant conditions. This normalization series will be a useful tool for such educational projects.
Supplementary Material
Acknowledgments
The authors thank Marie Birne, Arelys Uribe, and James Kwon for managing the fish facility; Jaymie Estevez for assistance with experiments; and Jenny Rynd and Sana Khan for helpful discussion. This study was supported by the Howard Hughes Medical Institute Summer Program for Undergraduate Students (to C.S.); the CUNY Graduate Center (to C.S.), NIH R03 HD055399, PSC-CUNY PSCREG-40-1135, and PSCREG-39-61492; the Fogel Fund and Queens College Research Enhancement funds (to N.G.H.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Marcus RC, Delaney CL, Easter SS., Jr.Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio). Vis Neurosci 1999;16:417–424 [DOI] [PubMed] [Google Scholar]
- 2.Gorelick DA, Watson W, Halpern ME. Androgen receptor gene expression in the developing and adult zebrafish brain. Dev Dyn 2008;237:2987–2995 [DOI] [PubMed] [Google Scholar]
- 3.Zupanc GK. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol Paris 2008;102:357–373 [DOI] [PubMed] [Google Scholar]
- 4.Kelsh RN, Harris ML, Colanesi S, Erickson CA. Stripes and belly-spots—a review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol 2009;20:90–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koide T, Miyasaka N, Morimoto K, Asakawa K, Urasaki A, Kawakami K, et al. . Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Natl Acad Sci U S A 2009;106:9884–9889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta V, Poss KD. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature 2012;484:479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singleman C, Holtzman NG. Analysis of post-embryonic heart development and maturation in the zebrafish, Danio rerio. Dev Dyn 2012;241:1993–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell 2012;22:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin VP, Lepilina A, Smith A, Poss KD. Regulation of zebrafish heart regeneration by miR-133. Dev Biol 2012;365:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuda J, Kearns-Sixsmith D. Outreach: empowering students and teachers to fish outside the box. Zebrafish 2009;6:133–138 [DOI] [PubMed] [Google Scholar]
- 11.Fields M. The art and science of zebrafish in the classroom—an interview with Melanie Fields, interviewed by Lara D. Hutson. Zebrafish 2009;6:121–126 [DOI] [PubMed] [Google Scholar]
- 12.Fields MC, Adelfio P, Ahmad D, Brown O, Cox B, Davies M, et al. . Danio rerio in K-12 classrooms: sparking interest in the new generation of scientists. Zebrafish 2009;6:145–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagatto B. Guided inquiry lab exercises in development and oxygen consumption using zebrafish. Zebrafish 2009;6:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duszynski RJ, Topczewski J, Leclair EE. Simple, economical heat-shock devices for zebrafish housing racks. Zebrafish 2011;8:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs NL, Albertson RC, Wiles JR. Using whole mount in situ hybridization to link molecular and organismal biology. J Vis Exp 2011;49:2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang JO, Abata K, Bachelder E, Bartley B, Bozadjieva N, Caskey V, et al. . Original research in the classroom: why do zebrafish spawn in the morning? Zebrafish 2011;8:191–202 [DOI] [PubMed] [Google Scholar]
- 17.Lindemann S, Senkler J, Auchter E, Liang JO. Using zebrafish to learn statistical analysis and Mendelian genetics. Zebrafish 2011;8:41–55 [DOI] [PubMed] [Google Scholar]
- 18.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 19.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 2009;238:2975–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol 2007;8:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushik S, Georga I, Koumoundouros G. Growth and body composition of zebrafish (Danio rerio) larvae fed a compound feed from first feeding onward: toward implications on nutrient requirements. Zebrafish 2011;8:87–95 [DOI] [PubMed] [Google Scholar]
- 22.Varga ZM. Aquaculture and husbandry at the zebrafish international resource center. Methods Cell Biol 2011;104:453–478 [DOI] [PubMed] [Google Scholar]
- 23.Tal TL, Franzosa JA, Tilton SC, Philbrick KA, Iwaniec UT, Turner RT, et al. . MicroRNAs control neurobehavioral development and function in zebrafish. FASEB J 2012;26:1452–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hisaoka KK. The effects of 2-acetylaminofluorene on the embryonic development of the zebrafish. II. Histochemical studies. Cancer Res 1958;18:664–667 [PubMed] [Google Scholar]
- 25.Hisaoka KK. The effects of 4-acetylaminofluorene on the embryonic development of the zebrafish. I. Morphological studies. Cancer Res 1958;18:527–535 [PubMed] [Google Scholar]
- 26.Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, et al. . Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep 2006;7:893–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabarti S, Streisinger G, Singer F, Walker C. Frequency of gamma-ray induced specific locus and recessive lethal mutations in mature germ cells of the zebrafish, Brachydanio rerio. Genetics 1983;103:109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quigley IK, Parichy DM. Pigment pattern formation in zebrafish: a model for developmental genetics and the evolution of form. Microsc Res Tech 2002;58:442–455 [DOI] [PubMed] [Google Scholar]
- 29.Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G. Changes in thyroid hormone levels during zebrafish development. Zool Sci 2012;29:181–184 [DOI] [PubMed] [Google Scholar]
- 30.Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci U S A 1997;94:13011–13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.