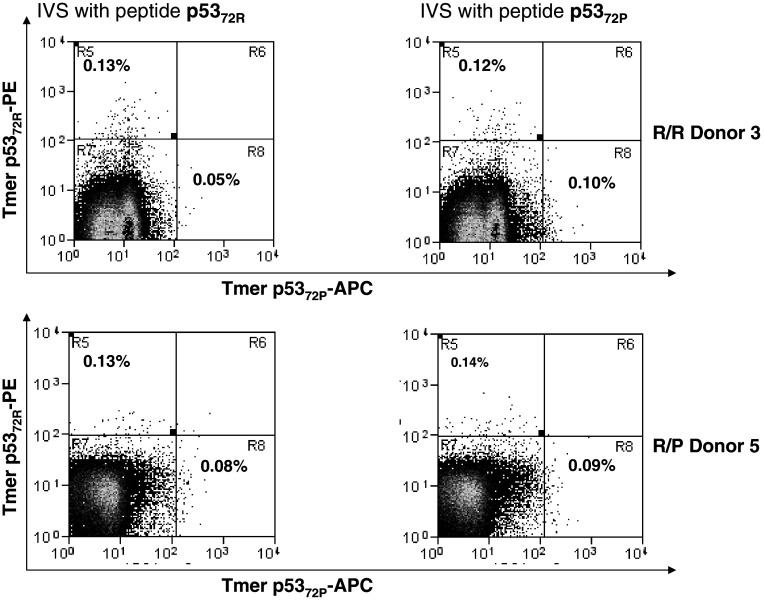
Fig. 4.
Frequency of wt p5372P and wt p5372R-specific tetramer+ cells in PBMC from healthy donors (HD) after 3-weeks in vitro stimulation (IVS). IVS was performed using autologous DC from HLA-A2+ HD loaded with wt p5372P and p5372R peptides (10µg/ml at 37°C for 4 h). Autologous CD8+ T cells were negatively isolated from PBMC with immunomagnetic beads (Miltenyi Biotech, Germany) and added at 1 × 106 cells/ml to 1 × 105 peptide-pulsed DC in a final volume of 2 ml of culture medium (24-well tissue culture plate). The cells were cultured for 7 days at 37°C with IL-2 (20 units/ml) and IL-7 (5 ng/ml). On day 7, lymphocytes were harvested and restimulated until the completion of 21 days. Two different genotypes (R/R and R/P) were tested for the specificity of PE-labeled HLA-A2-wt p5372R and APC-labeled HLA-A2-wt p5372P tetramer. The PE-labeled HLA-A2-wt p5372R and APC-labeled HLA-A2-wt p5372P tetramer were obtained from the Tetramer Facility of the National Institute of Allergy and Infectious Disease (Atlanta, GA, USA). Specificity was confirmed by staining of the CTL line specific for each peptide after IVS and the lack of staining of irrelevant (MAGE-3271–279 specific or HIV Pol 642A) CTL or HLA-A2− PBMC obtained from normal donors (not shown). Three-color flow cytometry assays (FACScan, BD Biosciences) were performed with fluorescent labeled anti-CD3 and FITC-anti-CD8 Abs (Beckman Coulter) and PE-tetramer or APC-tetramer. Flow cytometry was performed on a CyAn™ flow cytometer (Dako, Ft. Collins, CO, USA) machine, and data analyzed using Summit V4.3 software. Generally, 100,000 events per sample were collected after gating on lymphocytes by forward and side scatter