Abstract
Despite advances in patient and graft management, biliary complications (BC) still represent a challenge both in the early and delayed period after orthotopic liver transplantation (OLT). Because of unspecific clinical presentation, imaging is often mandatory in order to diagnose BC. Among imaging modalities, magnetic resonance cholangiography (MRC) has gained widespread acceptance as a tool to represent the reconstructed biliary tree noninvasively, using both the conventional technique (based on heavily T2-weighted sequences) and contrast-enhanced MRC (based on the acquisition of T1-weighted sequences after the administration of hepatobiliary contrast agents). On this basis, MRC is generally indicated to: (1) avoid unnecessary procedures of direct cholangiography in patients with a negative examination and/or identify alternative complications; and (2) provide a road map for interventional procedures or surgery. As illustrated in the review, MRC is accurate in the diagnosis of different types of biliary complications, including anastomotic strictures, non-anastomotic strictures, leakage and stones.
Keywords: Orthotopic liver transplantation, Orthotopic liver transplantation complications, Magnetic resonance imaging cholangiopancreatography, Endoscopic retrograde cholangiography, Bile ducts obstruction
Core tip: The review is focused on three main topics, in order to emphasize why magnetic resonance cholangiography (MRC) is the preferred imaging modality to noninvasively assess the biliary system after orthotopic liver transplantation. First, the authors describe the different techniques that can be used, namely conventional MRC and contrast-enhanced MRC. Second, exemplificative imaging findings are illustrated in order to show the diagnostic reliability of the technique. Third, the Authors discuss the state-of-the-art role for MRC in assessing biliary complications as emerging from updated literature review.
INTRODUCTION
Despite improvements in organ preservation, surgical technique, immunosuppression and postoperative management, biliary complications (BC) still represent the “Achille’s heel” of orthotopic liver transplantation (OLT), occurring in 10%-34% of graft recipients[1,2]. BC are associated with a significant morbidity and mortality rate (2%-7%)[3,4], representing the second leading cause of graft dysfunction and loss after rejection[1]. Prompt recognition or exclusion of BC is crucial in order to address patient to proper treatment. However, differentiating BC from other post-OLT complications can be difficult based solely on clinical presentation and biochemical findings, thus making imaging essential in the diagnostic process[5].
Among different imaging modalities, magnetic resonance cholangiography (MRC) plays a key role in evaluating BC after OLT. Due to the technical advances occurred over the last decades, MRC can be performed on magnetic resonance (MR) systems equipped with highly performing gradients, multichannel phased-array coils and dedicated sequences in order to produce panoramic and detailed representation of the biliary tree without significant motion-related artefacts[2]. Although it is questionable whether MRC can be viewed as the new standard of reference in biliary imaging[6], this technique has gained acceptance as the most reliable alternative to direct cholangiography in depicting the biliary system. In the setting of liver transplant, MRC is useful both in the pre- and post-operative period, e.g. in assessing the biliary anatomy of living donors[7,8]. Moreover, MRC is safe, repeatable and reproducible[7].
In this review, we: (1) describe different technical approaches to MRC; (2) discuss the evidence-based role of MRC in assessing BC after adult OLT; and (3) illustrate imaging findings of main BC. Although split-liver transplantation and LDTL are not directly discussed in this work, the paper statements on the use of MRC can be extended to these variants of transplantation.
CLINICAL OVERVIEW
Classification of biliary complications
BC can be classified according to the clinical phenotype, localization, timing of occurrence and etiology[5]. A useful classification for radiologists is based on the temporary onset from OLT, which is of help in identifying the most probable complication occurring at the time of image interpretation. Complications occurring within 3 mo after OLT are defined as “early”, and are typically represented by bile leakage and nonanastomotic strictures (NAS) related to hepatic artery thrombosis (HAT)[5]. “Late” complications occur a few months to several years later, and mainly consist in strictures. Anastomotic strictures (AS) show a tendency to develop earlier (within 4-5 mo) compared to non-HAT related NAS[5,9]. Overall, the large majority of BC (up to 80%) present within 6 mo from OLT[9], with annual incidence less than 4% after the first post-transplant year[10].
The characteristics of main BC are shown in Table 1, including the time of onset and main risk factors. Notably, split-liver transplant and LDLT have been associated with a moderate increase in BC, e.g., because of cut-surface leakage originating both in donors and recipients[11].
Table 1.
Overview of main biliary complications occurring after liver transplantation
| Type of complication | Prevalence in adult OLT patients | Risk factors | Time of onset from OLT | Clinical features | Treatment |
| Bile leak | 7.8% OLT 9.5% LDLT | T-tube displacement or removal (T-tube leak) technical failure during surgery (anastomotic leak) HAT (nonanastomotic leak) Ischemic-related injury, immunologically-related injury, cytotoxic injury induced by bile salts (nonanastomotic leak in pts. without HAT) | 1-3 mo | Fever, abdominal complaint, signs of cholestasis and or cholangitis | Leaving the T-tube open (T-Tube leaks) ERC with Sphincterotomy and stent placement Percutaneous drainage |
| Anastomotic stricture | 13% OLT 19% LDLT | Older donor age Roux-en-Y choledochojejunostomy Technical factors (earlier manifestation) Ischemia of the donor bile duct (earlier manifestation) Previous anastomotic leakage (late manifestation) | within 6 mo-1 yr, occasionally later | Biliary obstruction | Surgical revision (repair or conversion to bilio-enteric anastomosis) ERC with balloon dilatation and stent placement (usually repeated procedures) Surgical revision (conversion to bilio-enteric anastomosis) |
| NAS | 5%-25% | HAT Microangiopathic injury (prolonged warm or cold ischemia times of the graft) (ITBL) Immunogenic injury (AB0 incompatibility between donor and recipient, chronic ductopenic rejection, primitive sclerosing cholangitis) (ITBL) Cytotoxic injury by bile salts (ITBL) | Within 6 mo (HAT-associated NAS) After 6 mo (ITBL) | Cholestasis with recurrent cholangitis | Biliary toilette, dilatation ± stent placement via ERC/PTC Medical therapy (ursodeoxycholic acid and antibiotics if recurrent cholangitis) |
| Stones, casts and sludge | 5.70% | Anastomotic and nonanastomotic biliary strictures Presence of T-tube or stent Hepaticojejunostomy Ischemia Infectious alteration in bile composition | Within 1 yr (casts and sludge) After 1 yr (stones) | Biliary obstruction | Conversion to hepaticojejunostomy(rarely) Retransplantation Bile ducts toilette using ERC/PTC Medical therapy with ursodeoxycholic acid Retransplantation |
| Sphincter of Oddi dysfunction and papillary stenosis | 2%-7% | Denervation of the recipient common bile duct leading to sphincter of Oddi spasm Inflammation and/or scarring of the sphincter of Oddi | 6 mo to 1 yr | Increased cholestatic enzymes | Endoscopic sphincterotomy |
Biliary reconstruction
Prior to the examination, type of transplant (e.g., left/right split-liver transplant or living donor liver transplantation) and surgical technique should be evaluated in order to correctly interpret patient anatomy and MRC findings. Nowadays, biliary reconstruction during OLT is performed according to two main options[5] (Figure 1): (1) choledocho-choledochostomy, consisting in an end-to-end anastomosis between donor and recipient choledochal ducts (duct-to-duct technique); and (2) bilioenteric anastomosis, consisting in an end-to-side anastomosis between the donor hepatic duct and a recipient jejuneal loop (Roux-en-Y hepaticojejunostomy). Compared to bilioenteric anastomosis, duct-to-duct anastomosis is technically simpler and preserves the sphincter of Oddi as a barrier against bacterial colonization of the biliary tract[12]. This is why choledocho-choledochostomy is the preferred technique of biliary reconstruction. Bilioenteric anastomosis is usually reserved for cases of primary sclerosing cholangitis (PSC) as the indication to OLT, surgical salvage after BC or re-transplantation[12].
Figure 1.
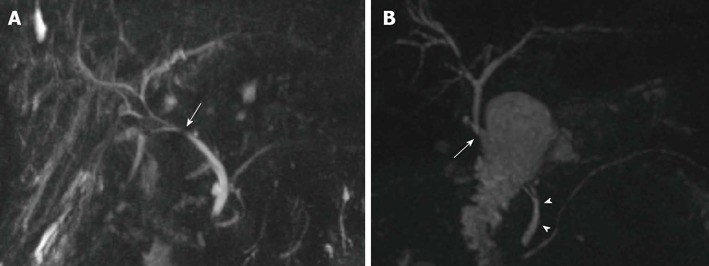
Biliary reconstructions variants after orthotopic liver transplantation illustrated by coronal maximum intensity projection reconstruction from 3D magnetic resonance cholangiography. A: Choledocho-choledochostomy with mild donor-to-recipient discrepancy in ductal calibers giving prominence to the anastomotic site (arrow); B: Bilioenteric anastomosis (arrow) between donor’s common bile duct and a jejuneal loop. Note the recipient common bile duct remnant (arrowheads).
The vascular supply of the biliary tract
Contrary to liver parenchyma, the biliary tree is nourished by arterial vessels only, which can be divided in two interconnected systems (Figure 2). The first one supplies the common bile duct and consist of the ascending axial branches originating from the gastroduodenal artery, which run on medial (“3 o’clock”) and lateral (“9 o’clock”) aspects of the common bile duct and communicate (usually) with the right hepatic artery. The second system is the peribiliary vascular plexus, which supplies the hepatic confluence and intrahepatic bile ducts. It consists of a complex arterial network originating from terminal arterial branches, being supported mainly through a “communicating arcade” running between hilar branches of the hepatic artery, with substantial anatomic variability[5,13].
Figure 2.
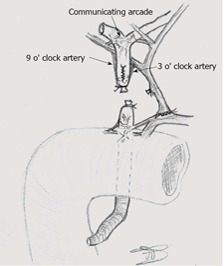
Arterial supply to the biliary tree in liver-transplanted patients.
Although surgical technique is aimed to preserve as much as possible biliary vascularization both in the donor and recipient, surgical sacrifice of arterial branches during the transplant make the bile ducts sensitive to “disconnection” from the hepatic artery and/or recipient gastroduodenal artery, as occurs during organ preservation or HAT. Hypoperfusion from HAT translates into ischemic cholangitis (“macroangiopathic” injury), with extensive bile epithelium necrosis, intraductal casts formation, bile leakage and evolution to scarring and multiple strictures, typically involving the hepatic confluence and intrahepatic bile ducts[9]. Surgical preservation of adequate perfusion at biliary ends and periductal tissue is also essential in reducing the risk of anastomotic stenosis[5]. Furthermore, hypoperfusion may result from a variety of transplant-related or immunologically-mediated causes (Table 1), causing “microangiopathic” ischemic damage of the peribiliary plexus. Such a damage translates into a macroscopic MRC pattern similar to that of macroangiopathic injury[5], as illustrated below.
Diagnostic approach to biliary complications
Clinical and laboratory diagnosis of BC is challenging, since manifestations such as fever, increase in bilirubin and altered liver function tests significantly overlap with other post-OLT entities, including rejection[9]. Of note, BC may co-exist with different types of complications or being a consequence of HAT, thus making differential diagnosis even more difficult. However, prompt recognition of primary or secondary biliary involvement is mandatory to allow proper treatment.
Ultrasound with color Doppler examination and/or contrast-enhanced ultrasound (CEUS) represent the first-line tool in excluding HAT as the primary source of BC and in assessing fluid collections suspicious for bilomas[14]. Despite the high negative predictive value (NPV) reported by some authors[15], US shows well known limitations in clinical practice, especially in the case of biliary obstruction. US lacks panoramicity and is often impaired by reduced patients’ compliance and presence of bowel gas and surgical dressing material. Additionally, the presence of epithelial casts filling the bile ducts in the postoperative period may further limit sonographic visibility[16]. As a consequence, it is difficult to establish the type and the site of the obstructive cause with US. Although biliary dilatation is a reliable indirect sign of biliary obstruction, biliary dilatation develops slowly and disproportionally with regard to the severity of the stricture[17], being undetectable in more than 60% with anastomotic stenosis[18]. In summary, normal US examination should not preclude further investigations in case of clinical suspicion.
According to Zoepf et al[16], Computed Tomography (CT) is able to show biliary dilatation in up to 40% and 83% of anastomotic and nonanastomotic strictures, respectively. However, because of limited contrast resolution and relative inability to show the anastomotic site, CT correctly identifies the site of biliary obstruction in 10% of patients only[2,16]. The main role for CT is then to assess HAT[19] and/or detect intra- and extra-hepatic hypoattenuating collections when a suspicious biloma has been raised by US.
The use of T-tube after OLT is still a matter for debate[5]. When available, T-tube cholangiography under fluoroscopic or CT guidance is a rapid and accurate tool to demonstrate the presence of bile leak during the limited period of time in which direct access to bile ducts is present (1-3 mo). According to Singh et al[20], T-tube cholangiography should be preferred over MRC because the distension of the bile ducts with contrast medium permits better stricture analysis and functional assessment. Therefore, once the T-tube is removed, alternative imaging methods must be used.
Because of the above limitations of US, CT and T-tube cholangiography, ERC and PTC are still considered the standard of reference in imaging patients with duct-to-duct anastomosis and bilioenteric anastomosis, respectively. The advantage of ERC and PTC is to allow interventional procedures such as sphincterotomy, ballooning or stenting, which are the first-line treatment of biliary obstruction. On the other hand, morbidity and mortality rates associated with direct cholangiography procedures have encouraged the use of MRC as the preferred, panoramic tool to assess BC, limiting ERC and PTC to interventional rather than diagnostic purpose[5,21]. We further discuss the role for ERC/PTC and MRC in the dedicated paragraph below, together with the advantages and disadvantages of these techniques.
Further investigations such as hepatobiliary scintigraphy provided controversial results, gaining no routine use[5]. On the contrary, liver biopsy is frequently necessary to establish final diagnosis underlying graft dysfunction[22], especially if microangiopathic biliary injury is suspected.
MRC TECHNIQUE
The goal of MRC is to provide a panoramic representation of hyperintense biliary tree against a low signal intensity background. Currently, two techniques are used to obtain images with such an elevated contrast, namely conventional MRC (C-MRC) and contrast-enhanced MRC (CE-MRC) (Figure 3). The difference between these techniques relies on the type of sequence, use of iv contrast agent, timing of acquisition and clinical indication.
Figure 3.
Technical variants of magnetic resonance cholangiography, as shown in coronal images of a 66-year-old male patient transplanted for alcoholic cirrhosis. A: Conventional, T2-weighted 2D MRC; B: MIP reconstruction from conventional, T2-weighted 3D MRC; C: Thick MIP reconstruction from T1-weighted, contrast-enhanced MRC. Both the degree and functional significance of the mild anastomotic stricture indicated by arrows are better showed by contrast passage in (C). MRC: Magnetic resonance cholangiography; MIP: Maximum intensity projection.
Regardless of the technique, MRC is rarely used as a standing-alone examination. MRI scanning protocols in post-OLT patients should always include non cholangiographic sequences in order to evaluate liver parenchyma and/or extrabiliary manifestations of BC such as bilomas and perihepatic free fluid[2].
Conventional MRC
In C-MRC, image contrast is the result of heavily T2-weighted sequences with a long TE. This emphasizes differences in transverse relaxation times between “slow motion” fluids such as the bile (long T2) and background tissues with intermediate to short T2[21]. In our Institution, we administer oral 1:10 mL water solution of a gadolinium chelate contrast agent just before the acquisition of MRC in order to suppress overlapping fluid signal from the stomach and/or duodenum with paramagnetic effects on the T2 relaxation time[23]. However, because of the risk to mask the vaterian region of the common bile duct as a possible site of BC, the use of oral negative contrast agent is a matter of expertise and institutional preferences. Based on the inherent high contrast of C-MRC, no i.v. administration of gadolinium-based contrast agents is needed, which is of relevance in patients with renal function impairment at risk of developing Nephrogenic Systemic Fibrosis (NSF)[24].
C-MRC can be performed with the 3D and/or 2D approach choosing among a variety of well-established MRC sequences (Figure 3). The 3D technique is usually based on respiratory-triggered or navigator-gated volumetric Turbo/Fast Spin Echo sequences acquired during normal patient respiration, and provides numerous thin slices with higher signal-to-noise ratio and spatial resolution as a base for multiplanar reformations ad maximum intensity projection (MIP) reconstructions[25]. Compared to the 2D variant, 3D C-MRC has the advantage of higher longitudinal spatial resolution, with the capability of achieving isotropic imaging and assessing more subtle anatomic and pathological details, such as small calculi[25]. On the other hand, 3D C-MRC can be significantly affected by motion artifacts in non-collaborating patients. The 2D technique is acquired more rapidly, during few and short breath-holds using thick slices, thus reducing the effects of respiratory artefacts on image quality[26]. Different sequences are currently available to perform 2D C-MRC, including RARE (rapid acquisition with relaxation enhancement), HASTE (half-Fourier acquisition single-shot turbo spic echo) and SS-F/TSE (single-shot fast/turbo spin echo)[26].
To our knowledge, only a few studies by Kinner et al[26,27] compared the 2D and 3D techniques in assessing BC after OLT. According to these Authors, overall image quality and accuracy are comparable in patients with biliary obstruction, regardless of the C-MRC sequence. However, the 3D technique shows slight better diagnostic performance in assessing BC, especially in the case of patients with choledocho-choledochostomy and biliary strictures[27]. Although the use of the 2D or 3D technique depends on institutional preferences, both approaches should be used in the standard examination, in order to exploit the advantages and counterbalance the drawbacks of each technique.
Contrast-enhanced MRC
Over the last years, there has been an increasing interest in the use of CE-MRC in the post-surgical assessment of the biliary tree[28]. This technique is based on i.v. administration of hepatospecific contrast agents such as gadoxetic acid (Gd-EOB-DTPA), gadobenate dimeglumine (Gd-BOPTA)[29] or mangafodipir trisodium[30], that are excreted into the bile after hepatocellular uptake, thus complementing morphological C-MRC with information on the bile flow. In our experience, the most suitable contrast agent in this setting is gadoxetic acid, because of larger hepatocellular uptake (50% of the administered dose) and the relatively short time to achieve the hepatobiliary phase, i.e., 10 to 20 min after contrast administration in patients with preserved liver function[31]. As T2-shortening effects might mask the biliary tree on T2-weighted images, it is mandatory to perform CE-MRC after C-MRC, using a volumetric, high-resolution T1-weighted 3D fat-satured sequence)[31]. The use of larger flip angles (e.g., 35°) is recommended in order to increase the conspicuity of the biliary tree over the background[32].
Despite the increasing use of CE-MRC, there is a paucity of literature-based evidence in the setting of OLT, mainly focused on the preoperative evaluation of liver donors[33,34]. However, studies on patients with BC after hepatobiliary surgery, including OLT subjects, suggest that CE-MRC improves the accuracy of C-MRC in evaluating bile leakage showing the site of the leak and direct contrast extravasation into perihepatic/peribiliary fluid collections[35]. Moreover, CE-MRC is indicated to “functionally” assess the degree of biliary obstruction according to the presence or absence of the contrast medium downstream in the bile duct (Figure 3). This is of importance in patients with bilioenteric anastomosis, since the diagnosis of anastomotic stricture can be difficult even in the presence of biliary dilatation[31]. In our experience, the degree of contrast flow is helpful (1) in the distinction between “normal” scarring of the anastomotic site and obstructive anastomotic stenosis in patients with choledocho-coledochostomy; or (2) in the assessment of diffuse bile ducts damage in the case of bile casts syndrome.
Hepatocellular uptake of gadoxetic acid is mediated by the same anionic transporter of bilirubin. As a consequence, biliary excretion of gadoxetic acid is limited or delayed by impaired liver function[35]. Although impaired biliary excretion can be used as an indirect sign of biliary obstruction[31,35], this translates into reduced visualization of the bile ducts or the need to perform delayed image acquisitions up to 90-180 min after contrast administration[31]. In our opinion, the costs inherent to contrast agents imply that CE-MRC should be used to complement C-MRC when “functional” information is needed, after careful evaluation of patients liver function. In our Institution, we avoid CE-MRC when bilirubin level is higher than 5 mg/dL.
MRC FINDINGS
Normal findings after OLT
Normal post-OLT Imaging findings mirror some “physiologic” effects of the surgical procedure. Not surprisingly, then, it is frequent to observe small amounts of free fluid or small fluid collections in the perihepatic region, intersegmental fissure and subhepatic space, as well as along the resection margin after split liver-OLT and LDLT[36]. Clinical and biochemical correlation is helpful in order not to misinterpret these findings as bilomas. Collections tend to resolve spontaneously after few weeks from the intervention[20].
Mild anastomotic narrowing with minimal concentric wall thickening of the common bile duct is a frequent MRC finding[2] that should be interpreted as normal, unless biliary dilatation upstream and symptoms of biliary origin are present[37]. In most cases, anastomotic narrowing is the effect of surrounding edema, resolving during the first weeks after OLT[11]. In our experience, narrowing or kinking of the common bile duct at the anastomotic site are common findings, especially in the case of redundancy or disproportion between the donor and recipient common bile ducts[3]. These conditions are useful in identifying the site of anastomosis in the case of choledocho-choledochostomy, and should not be assessed as a complication unless biliary obstruction is associated (Figure 1).
Bile leakage
Leakage represents the most common early biliary complication. In up to 80% of patients with leakage[9], leaks manifest at the insertion of the T-tube, usually as a consequence of dislocation or after the removal of the device[38]. Other sites include: (1) the biliary anastomosis or cystic duct remnant, as an effect of technical failure[11]; (2) the cut-surface after split-liver OLT or LDLT, possibly in relation to patent or aberrant bile ducts and necrosis of liver tissue[5]; and (3) wherever along the biliary tree (intrahepatic and/or extrahepatic bile leakage) because of bile ducts ischemia after HAT (see above).
On T2-weighted C-MRC, biliary leakage manifests indirectly as bilomas, i.e., a well-delineated fluid collections lying in the perihilar or subhepatic space, as well as along the resection margin in LDLT or split-liver OLT. A variable amount of free bile can be associated around the perihepatic space or intersegmental fissure. These findings can be indistinguishable from normal postoperative free fluid or collections. Bilomas can be suspected when a thin, hyperintense direct communication between a fluid collection and the T-tube entry site and/or biliary anastomosis is shown[11] (Figure 4). When leakage is suspected, CE-MRC is an effective complement to C-MRC in order to demonstrate both the site of contrast extravasation and contrast transit into the biloma or free fluid, with sensitivity for combined C-MRC and CE-MRC of 84%[35]. Confirmation of diagnosis is usually obtained during therapeutic ERC.
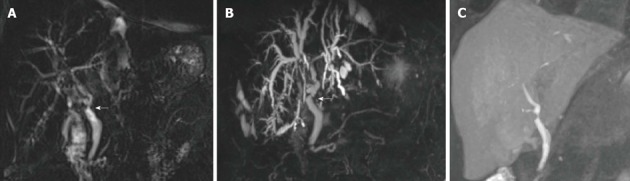
Figure 4.
Bile leakage in a 54-year-old male subject transplanted for hepatitis C virus-related cirrhosis. Perihilar biloma shown by arrowheads on coronal T2-weighted HASTE image (A) and paracoronal MIP reconstruction from 3D MRC (B). Thin communication between the anastomotic site and fluid collection is visible on the axially-reformatted 3D source image (arrow in C). MRC: Magnetic resonance cholangiography; MIP: Maximum intensity projection.
Strictures
Strictures can be classified into anastomotic strictures (AS) or nonanastomotic strictures (NAS) according to the site of manifestation, which reflects different pathological mechanisms of origin (Table 1). NAS are further differentiated into forms associated with HAT (macroangiopathic damage) and forms occurring during later from OLT, in the presence of patent hepatic artery (microangiopathic damage). Non-HAT associated NAS are overall categorized as Ischemic-type biliary lesions (ITBL)[5,9,11].
Anastomotic strictures
In patients with choledocho-choledochostomy, luminal narrowing manifests as a focal tract of decreased or absent bile signal intensity of the reconstructed common bile duct, lying between donor and recipient remnants of the cystic duct. A variable degree of common bile duct angulation can be associated[11]. AS can be classified as mild, moderate or severe. Of note, MIP reconstructions from 3D C-MRC tend to overestimate the degree of luminal narrowing compared to ERC[39,40]. Consequently, 3D source data and/or 2D MRC should always be evaluated when assessing strictures, although functional effects on the bile flow are easily inferred by the degree (1) of the associated suprastenotic biliary dilatation; and (2) contrast passage downward when using CE-MRC.
In the case of bilioenteric AS, luminal narrowing appears as a focal absence of biliary signal in the segment immediately above the jejuneal loop, corresponding to low-signal thickening of the common bile duct on axial or axially-reformatted 3D images[2,41]. However, the accuracy of C-MRC in evaluating AS is lower in patients with bilioenteric anastomoses compared to those with choledocho-choledochostomy, regardless of the use of 2D or 3D technique[27]. This difference has been explained by the difficulty in correctly identifying the anastomotic site, which is partially masked by the hyperintense fluid content of the anastomotic bowel tract. Furthermore, mild duct dilatation is frequently present in patients with patent bilioenteric anastomosis due to physiological changes in caliber as the bile duct enters the bowel wall[26] or temporary folding of the anastomotic site caused by the anastomotic loop motility[41]. CE-MRC with delayed imaging has the potential to clearly define the presence of biliary obstruction, thus avoiding false-positive results. Based on the degree of contrast transit at 30 min from contrast injection, the degree of bile duct obstruction can be classified as[31]: (1) complete (absence of contrast filling in the proximal part of the stricture); (2) near-complete (significantly delayed contrast agent filling only in the proximal part of the stricture); and (3) partial (passage of contrast agent beyond the stricture).
Nonanastomotic strictures
Regardless of the timing of onset and different pathogenic mechanism (Table 1), NAS related to HAT and ITBL manifest with a similar pattern of extensive biliary injury, consisting in irregularly marginated bile ducts with multiple focal stenoses typically involving the hepatic confluence (with the hepatic duct) and/or intrahepatic bile ducts (Figure 5). Bile ducts segments above or between strictures show a variable degree of biliary dilatation upstream[11]. The involvement of intrahepatic ducts equal or larger than the second-order should be clearly identified, because this findings is associated with worst response to therapy[42]. The involvement of small peripheral ducts (third-order or larger) is typical of microangiopathic forms of NAS, frequently evolving to ducts rarefaction over time[5]. Potential disadvantages of MRC are the difficulty in establishing the degree and length of dominant strictures[43], as well as the identification of subtle alterations of peripheral bile ducts[27]. Additionally findings of NAS include (Figure 6): (1) intrahepatic or extrahepatic bilomas in HAT-related forms, as a consequence of the necrosis of bile duct walls[11]; (2) ischemic damage of liver parenchyma; and (3) casts and sludge filling bile ducts, originated by the aggregation between bile products and desquamated epithelial cells[5].
Figure 5.
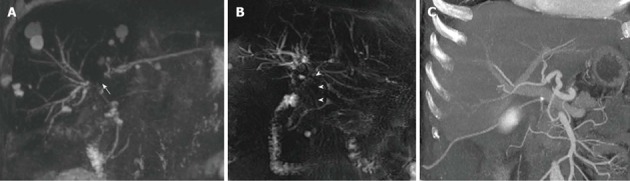
Nonanastomotic strictures in two different transplanted patients. A: MIP reconstruction shows early effects of HAT in a 58-year-old subject with hepatitis B virus infection, consisting in a stricture of the hepatic confluence (arrow) and multiple intrahepatic bilomas; B: ITBL in a 68 male patient transplanted for alcoholic cirrhosis. 2D MRC image shows a stricture of the hepatic confluence extended to the donor common hepatic duct (arrowheads); C: CT angiography found patent hepatic artery in this patient. MRC: Magnetic resonance cholangiography; MIP: Maximum intensity projection; ITBL: Ischemic-type biliary lesions.
Figure 6.
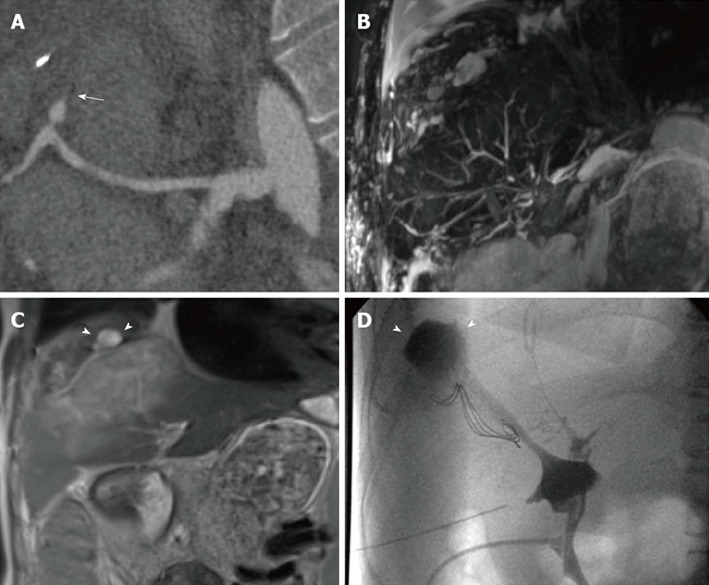
Multiple findings in a 28-year-old female patient transplanted for primary sclerosing cholangitis. Because of the hepatic artery thrombosis shown on curved-reformatted CT image (arrow in A), the biliary tree appears as fragmented and anatomically ill-defined on a panoramic maximum intensity projection view (B). Coronal T2-weighted HASTE image (C) shows extensive, hyperintense ischemic damage of liver parenchyma, together with intrahepatic fluid collections (arrowheads) confirmed to be the effect of bile leakage on T-tube cholangiography (arrowheads in D).
Recurrent PSC and biliary involvement secondary to chronic rejection may mimic NAS. In particular, chronic rejection has been associated with diffuse “vanishing bile duct” appearance involving more peripheral intrahepatic branches, thus showing some aspects in common with late NAS[11]. Timing of presentation, clinical history and results of liver biopsy are helpful in performing differential diagnosis.
Other complications
Sludge, casts and stones: Sludge, casts and stones are usually a concomitant manifestation of AS and NAS, showing a common appearance of an intense filling defects surrounded by a thin rim of bile signal. Typical locations include larger intrahepatic ducts[2] or the common bile duct immediately above a stricture. Usually, stones form later than sludge and casts, showing more rounded shape and smooth margins (Figure 7)[11]. Casts can be extensively distributed along biliary branches, obscuring the visibility of the hyperintense bile on C-MRC images. The only indirect sign of casts can be intermediate signal on T1- and T2-weighted noncholangiographic images with portal distribution, with periportal enhancement on postcontrast images due inflammation of the peribiliary space[44]. Notably, cast can accumulate extensively in the so-called “biliary-cast syndrome” (BCS), in which hardened, lithogenic material occupies the biliary ductal system shaping on the bile ducts, regardless of ischemic injury[45]. Since diagnosis of BCS and smaller filling defects is challenging on C-MRC, the use of CE-MRC has been advocated as a useful tool to improve diagnostic accuracy[46].
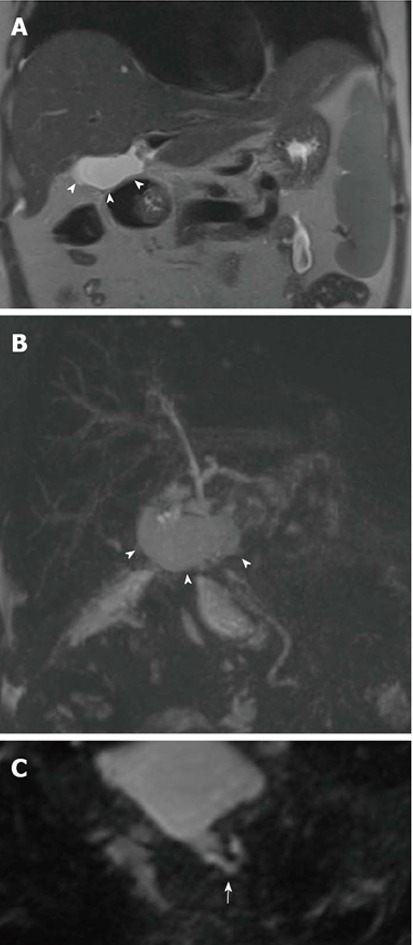
Figure 7.
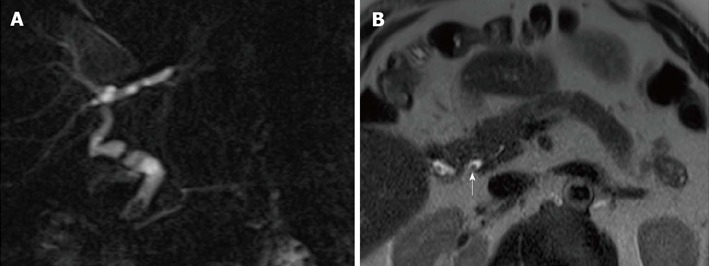
Calculi in a 62-year-old male subject who underwent orthotopic liver transplantation for hepatitis C virus-infection and hepatocellular carcinoma. A: The patient shows chronic kinking and moderate anastomotic stricture without biliary obstruction; B: Filling defect visible in the distal common bile duct were confirmed on axial HASTE image (arrow) and proven to be small calculi on ERC.
Differential diagnosis with stones, sludge and casts mainly includes aerobilia. Aerobilia is frequent after ERC or in patients with bilioenteric anastomosis. Air bubbles usually form an air-fluid level on axial images[11] (Figure 8) and can be associated with characteristic magnetic susceptibility artefact on noncholangiographic images obtained with GRE T1-weighted or Diffusion-weighted sequences (Figure 9).
Figure 8.
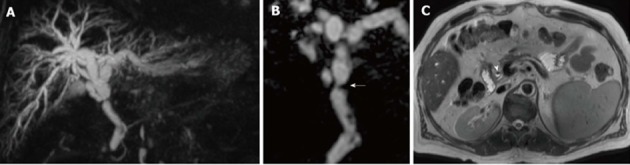
Anastomotic stricture in a 54-year-old male patient who underwent orthotopic liver transplantation for alcoholic cirrhosis. A: Coronal MIP reconstruction shows the stricture at the middle third of the extrahepatic bile duct, with biliary dilatation upstream; B: The degree of the stricture is better delineated on the paracoronally-reformatted thin 3D image (arrow); C: Filling defects visible on MRC images correspond to pneumobilia, appearing as air-fluid levels (arrowhead) on the axial T2-weighted HASTE sequence. MRC: Magnetic resonance cholangiography; MIP: Maximum intensity projection.
Figure 9.
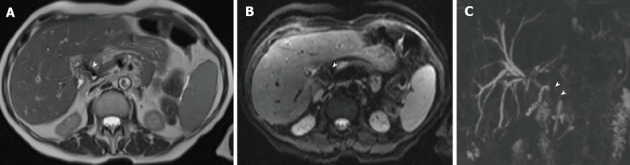
Aerobilia in a 62-year-old female patient transplanted for alcoholic cirrhosis. Anintense filling defect in the common bile duct on T2-weighted axial HASTE image (arrowhead in A) is associated to distortion artifact on axial Diffusion-weighted sequence (arrowhead in B). The effect of pneumobilia was to extensively mask the common bile duct on 3D magnetic resonance cholangiography (arrowheads in C).
Sphincter of Oddi dysfunction and papillary stricture: Distal obstruction of the common bile ducts usually translates into significant biliary dilatation of the recipient portion of the extrahepatic bile duct, although dilatation can rarely extends to intrahepatic bile ducts. In our experience, serial acquisition of “cinematic” 2D MRC images are useful in establishing the diagnosis, showing persistent lack of visualization of the vaterian sphincter tract of the common bile duct, suggesting spasm or stenosis (Figure 10). Final diagnosis is usually obtained with ERC or manomentry of the sphincter of Oddi[11].
Figure 10.
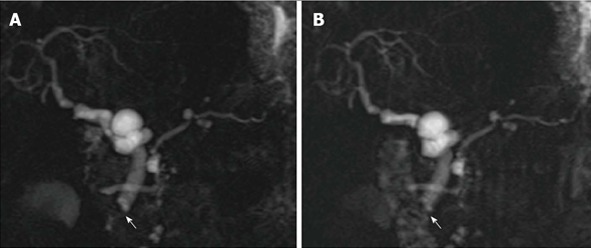
Sphincter of Oddi dysfunction in a liver-transplanted female patient with cholestasis and abdominal complaint years after orthotopic liver transplantation. Serial 2D cinematic magnetic resonance cholangiography images (A) and (B) acquired after few seconds show redundancy of the reconstructed common bile duct, which appear slightly dilated in the recipient tract, and persistent lack of visualization of the vaterian sphincter complex, with typical “meniscus sign” (arrow) suggesting spasm.
ROLE FOR MRC IN PATIENT’S MANAGEMENT
ERC still represents the standard of reference for biliary obstruction complicating OLT[1]. One might conclude that patients with suspicious BC should undergo ERC after preliminary US and/or CT evaluation. On the other hand, ERC is associated with a significant risk of pancreatitis, bleeding, infection, perforation and sedation-related complications, with morbidity and mortality rates of 10% and 0.5%, respectively[1]. The risk of complications is even higher when using PTC. Thus, the risk profile for diagnostic procedures of direct cholangiography seems not justifiable given the high diagnostic accuracy of MRC, which shows 97% sensitivity and 98% specificity for biliary obstruction according to a recent metanalysis[47]. Unfortunately, there is a relative paucity of studies[3,21,43,48-53] investigating the role for MRC in the specific setting of post-OLT BC, leading to difficulties in generalizing results from general population to transplant recipients[1]. For instance, some authors[47] have hypothesized that reduced biliary dilatation following post-OLT strictures might limit the accuracy of MRC. Detractors of MRC also argue that, although MRC correlates well with direct cholangiography procedures (P = 0.01)[54], the examination delays the diagnosis when interventional ERC or PTC are finally needed. This is why the use of MRC still depends on local preferences based on availability, expertise and costs.
Based on the above premises, one might ask which evidence-based task can be reasonably attributed to MRC in patients management. Table 2 shows the results of the two systematic reviews[1,55] focusing on this topic. Interestingly, Jorgensen et al[1] provide indirect information on the role for MRC by hypothesizing clinical scenarios with pre-test probability of BC of 25% and 50%, respectively. In the case of positive MRC, the post-test probability of BC reaches 80% and 94%, respectively, whereas in the case of negative MRC, the post-test probability reduces to 1% and 4%, respectively. These estimates emphasize the results of previous direct comparison between MRC, ERC and PTC[54], suggesting that the strength of MRC is represented by the large negative predictive value (94.4%), which is of help in excluding BC and avoiding unnecessary invasive procedures in patients with clinical low-to-moderate risk of BC[54]. Unfortunately, several methodological flaws affect the studies included in the above systematic reviews, including small sample size, uncertainty in clinical criteria defining the suspicion for BC, verification bias given the heterogeneity in the standard of reference tools and absence of a standardized MRC technique[1,55]. This is why the increasing (and reasonable) practice of using MRC as a screening tool for BC should be more adequately supported by: (1) prospective, large studies performed on patients initially assessed as having low-to-moderate risk for BC; (2) studies of cost-effectiveness on the systematic use of MRC in this category of patients.
Table 2.
Results of previous systematic reviews on the role for magnetic resonance cholangiography in assessing biliary complications after orthotopic liver transplantation
| Jorgensen et al[1] |
Xu et al[55] |
||
| Goal | Biliary obstruction | All biliary complications | Subset of strictures |
| Pooled sensitivity | 96.0% (0.92%-0.98%) | 0.95% (0.92%-0.97%) | 0.94% (0.88%-0.98%) |
| Pooled specificity | 0.94% (0.90%-0.97%) | 0.92% (0.89%-0.94%) | 0.95% (0.88%-0.99%) |
| AUC | 0.99 | 0.97 | 0.97 |
| Pooled PLR | 17.00 (9.4-29.6) | 10.23 (6.21-16.84) | 9.96 (2.52-39.36) |
| Pooled NLR | 0.04 (0.02-0.08) | 0.08 (0.06-0.12) | 0.09 (0.04-0.17) |
Number between parentheses represent the 95%CI. Analysis by Xu et al is stratified for the whole of complications and the subset of strictures. AUC: Area under the curve at Summary Receiving Operating Characteristic (SROC) curve; PLR: Positive likelihood ratio; NLR: Negative likelihood ratio.
On the other hand, it should be emphasized that a positive MRC examination cannot be simply considered as a cause of diagnostic delay. Differently from ERC and PTC, C-MRC depicts the bile ducts: (1) in their normal state, rather than artificially dilated by contrast injection pressure; and (2) below and above obstruction sites[54], thus making visible the whole biliary tract, regardless of impaired contrast passage. CE-MRC can complement this panoramic information as illustrated above. As a consequence, a positive MRC examination provides a road-map useful to plan better interventional or surgical approach, thus potentially contributing to reduce morbidity related to invasive procedures.
CONCLUSION
MRC has gained widespread acceptance as a tool to panoramically and reliably represent the biliary tree in post-OLT patients with suspected BC. Conventional technique, based on 2D or 3D heavily T2-weighted sequences, can be now complemented by CE-MRC using hepatospecific contrast agents, thus adding functional information to the morphological depiction of bile ducts. Although a consensus on the best study protocol is still lacking, a combination of the available technique is reasonably the best choice to enhance the diagnostic capabilities of MRC.
Because of the inherent high contrast of bile ducts, MRC has the capability to reliably identify most relevant BC, including bile leakage, AS, NAS and a variety of further disorders including calculi or sphincter of Oddi dysfunction. However, concerns still exist regarding the cost-effectiveness of this imaging modality in the everyday clinical practice, since positive MRC examinations often lead to ERC and PTC, which are still considered as the standard of reference for final diagnosis. A review of the literature suggests that, despite the absence of large multicentric trials on proper target populations, the high negative predictive of MRC is of value in excluding BC in patients with low-to-moderate risk, thus avoiding unnecessary invasive procedures. On the other hand, positive MRC provides a detailed road-map for interventional procedures or surgery, thus further contributing to reduce morbidity.
In summary, MRC is gaining an increasing role in the diagnosis and management of BC after OLT, and should be performed confidently in patients with low-to intermediate risk of disease.
ACKNOWLEDGMENTS
The authors thank: (1) Dr. Paolo Divis for having edited the images and drawn Figure 2; (2) Dr. Iliana and Sandra Bednarova’ for having revised English language.
Footnotes
P- Reviewer: Dirchwolf M, Maurea S, Radmard AR S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, Singal AG, Taylor JR, Elmunzer BJ. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. Gastrointest Endosc. 2011;73:955–962. doi: 10.1016/j.gie.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecchi A, De Santis M, Di Benedetto F, Gibertini M, Gerunda G, Torricelli P. Role of magnetic resonance cholangiography in biliary complications of orthotopic liver transplantation. Radiol Med. 2010;115:1065–1079. doi: 10.1007/s11547-010-0563-7. [DOI] [PubMed] [Google Scholar]
- 3.Valls C, Alba E, Cruz M, Figueras J, Andía E, Sanchez A, Lladó L, Serrano T. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol. 2005;184:812–820. doi: 10.2214/ajr.184.3.01840812. [DOI] [PubMed] [Google Scholar]
- 4.Boraschi P, Donati F. Complications of orthotopic liver transplantation: imaging findings. Abdom Imaging. 2004;29:189–202. doi: 10.1007/s00261-003-0109-8. [DOI] [PubMed] [Google Scholar]
- 5.Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253–265. doi: 10.1111/ajt.12034. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugam V, Beattie GC, Yule SR, Reid W, Loudon MA. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br J Radiol. 2005;78:888–893. doi: 10.1259/bjr/51075444. [DOI] [PubMed] [Google Scholar]
- 7.Sirvanci M, Duran C, Ozturk E, Balci D, Dayangaç M, Onat L, Yüzer Y, Tokat Y, Killi R. The value of magnetic resonance cholangiography in the preoperative assessment of living liver donors. Clin Imaging. 2007;31:401–405. doi: 10.1016/j.clinimag.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.An SK, Lee JM, Suh KS, Lee NJ, Kim SH, Kim YJ, Han JK, Choi BI. Gadobenate dimeglumine-enhanced liver MRI as the sole preoperative imaging technique: a prospective study of living liver donors. AJR Am J Roentgenol. 2006;187:1223–1233. doi: 10.2214/AJR.05.0584. [DOI] [PubMed] [Google Scholar]
- 9.Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;(243):89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 10.Brown RS, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 11.Girometti R, Cereser L, Como G, Zuiani C, Bazzocchi M. Biliary complications after orthotopic liver transplantation: MRCP findings. Abdom Imaging. 2008;33:542–554. doi: 10.1007/s00261-007-9316-z. [DOI] [PubMed] [Google Scholar]
- 12.García-Criado A, Gilabert R, Bargalló X, Brú C. Radiology in liver transplantation. Semin Ultrasound CT MR. 2002;23:114–129. doi: 10.1016/s0887-2171(02)90032-6. [DOI] [PubMed] [Google Scholar]
- 13.Gunji H, Cho A, Tohma T, Okazumi S, Makino H, Shuto K, Mochizuki R, Matsubara K, Hayano K, Mori C, et al. The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg. 2006;192:276–280. doi: 10.1016/j.amjsurg.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Hussaini SH, Sheridan MB, Davies M. The predictive value of transabdominal ultrasonography in the diagnosis of biliary tract complications after orthotopic liver transplantation. Gut. 1999;45:900–903. doi: 10.1136/gut.45.6.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoepf T, Maldonado-Lopez EJ, Hilgard P, Dechêne A, Malago M, Broelsch CE, Schlaak J, Gerken G. Diagnosis of biliary strictures after liver transplantation: which is the best tool? World J Gastroenterol. 2005;11:2945–2948. doi: 10.3748/wjg.v11.i19.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girometti R, Molinari C, Del Pin M, Toniutto P, Bitetto D, Como G, Zuiani C, Bazzocchi M. Degree of bile-duct dilatation in liver-transplanted patients with biliary stricture: a magnetic resonance cholangiography-based study. Radiol Med. 2012;117:1097–1111. doi: 10.1007/s11547-012-0805-1. [DOI] [PubMed] [Google Scholar]
- 18.Zemel G, Zajko AB, Skolnick ML, Bron KM, Campbell WL. The role of sonography and transhepatic cholangiography in the diagnosis of biliary complications after liver transplantation. AJR Am J Roentgenol. 1988;151:943–946. doi: 10.2214/ajr.151.5.943. [DOI] [PubMed] [Google Scholar]
- 19.Katyal S, Oliver JH, Buck DG, Federle MP. Detection of vascular complications after liver transplantation: early experience in multislice CT angiography with volume rendering. AJR Am J Roentgenol. 2000;175:1735–1739. doi: 10.2214/ajr.175.6.1751735. [DOI] [PubMed] [Google Scholar]
- 20.Singh AK, Nachiappan AC, Verma HA, Uppot RN, Blake MA, Saini S, Boland GW. Postoperative imaging in liver transplantation: what radiologists should know. Radiographics. 2010;30:339–351. doi: 10.1148/rg.302095124. [DOI] [PubMed] [Google Scholar]
- 21.Fulcher AS, Turner MA. Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology. 1999;211:715–722. doi: 10.1148/radiology.211.3.r99jn17715. [DOI] [PubMed] [Google Scholar]
- 22.Desai M, Neuberger J. Chronic liver allograft dysfunction. Transplant Proc. 2009;41:773–776. doi: 10.1016/j.transproceed.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Chan JH, Tsui EY, Yuen MK, Szeto ML, Luk SH, Wong KP, Wong NO. Gadopentetate dimeglumine as an oral negative gastrointestinal contrast agent for MRCP. Abdom Imaging. 2000;25:405–408. doi: 10.1007/s002610000018. [DOI] [PubMed] [Google Scholar]
- 24.Chow DS, Bahrami S, Raman SS, Rotchel S, Sayre JW, Busuttil RW, Lu DS. Risk of nephrogenic systemic fibrosis in liver transplantation patients. AJR Am J Roentgenol. 2011;197:658–662. doi: 10.2214/AJR.10.5976. [DOI] [PubMed] [Google Scholar]
- 25.Nandalur KR, Hussain HK, Weadock WJ, Wamsteker EJ, Johnson TD, Khan AS, D’Amico AR, Ford MK, Nandalur SR, Chenevert TL. Possible biliary disease: diagnostic performance of high-spatial-resolution isotropic 3D T2-weighted MRCP. Radiology. 2008;249:883–890. doi: 10.1148/radiol.2493080389. [DOI] [PubMed] [Google Scholar]
- 26.Kinner S, Dechêne A, Ladd SC, Zöpf T, de Dechêne EM, Gerken G, Lauenstein TC. Comparison of different MRCP techniques for the depiction of biliary complications after liver transplantation. Eur Radiol. 2010;20:1749–1756. doi: 10.1007/s00330-010-1714-x. [DOI] [PubMed] [Google Scholar]
- 27.Kinner S, Dechêne A, Paul A, Umutlu L, Ladd SC, de Dechêne EM, Zöpf T, Gerken G, Lauenstein TC. Detection of biliary stenoses in patients after liver transplantation: is there a different diagnostic accuracy of MRCP depending on the type of biliary anastomosis? Eur J Radiol. 2011;80:e20–e28. doi: 10.1016/j.ejrad.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Salvolini L, Urbinati C, Valeri G, Ferrara C, Giovagnoni A. Contrast-enhanced MR cholangiography (MRCP) with GD-EOB-DTPA in evaluating biliary complications after surgery. Radiol Med. 2012;117:354–368. doi: 10.1007/s11547-011-0731-4. [DOI] [PubMed] [Google Scholar]
- 29.Ergen FB, Akata D, Sarikaya B, Kerimoglu U, Hayran M, Akhan O, Hussain HK. Visualization of the biliary tract using gadobenate dimeglumine: preliminary findings. J Comput Assist Tomogr. 2008;32:54–60. doi: 10.1097/RCT.0b013e3180616b87. [DOI] [PubMed] [Google Scholar]
- 30.Fayad LM, Holland GA, Bergin D, Iqbal N, Parker L, Curcillo PG, Kowalski TE, Park P, Intenzo C, Mitchell DG. Functional magnetic resonance cholangiography (fMRC) of the gallbladder and biliary tree with contrast-enhanced magnetic resonance cholangiography. J Magn Reson Imaging. 2003;18:449–460. doi: 10.1002/jmri.10369. [DOI] [PubMed] [Google Scholar]
- 31.Boraschi P, Donati F. Biliary-enteric anastomoses: spectrum of findings on Gd-EOB-DTPA-enhanced MR cholangiography. Abdom Imaging. 2013;38:1351–1359. doi: 10.1007/s00261-013-0007-7. [DOI] [PubMed] [Google Scholar]
- 32.Stelter L, Grieser C, Fernándes CM, Rothe JH, Streitparth F, Seehofer D, Hamm B, Denecke T. Flip angle modulations in late phase Gd-EOB-DTPA MRI improve the identification of the biliary system. Eur J Radiol. 2012;81:e991–e995. doi: 10.1016/j.ejrad.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Mangold S, Bretschneider C, Fenchel M, Seeger A, Kramer U, Klumpp B, Nadalin S, Königsrainer A, Claussen CD, Miller S. MRI for evaluation of potential living liver donors: a new approach including contrast-enhanced magnetic resonance cholangiography. Abdom Imaging. 2012;37:244–251. doi: 10.1007/s00261-011-9736-7. [DOI] [PubMed] [Google Scholar]
- 34.Lee MS, Lee JY, Kim SH, Park HS, Kim SH, Lee JM, Han JK, Choi BI. Gadoxetic acid disodium-enhanced magnetic resonance imaging for biliary and vascular evaluations in preoperative living liver donors: comparison with gadobenate dimeglumine-enhanced MRI. J Magn Reson Imaging. 2011;33:149–159. doi: 10.1002/jmri.22429. [DOI] [PubMed] [Google Scholar]
- 35.Kantarcı M, Pirimoglu B, Karabulut N, Bayraktutan U, Ogul H, Ozturk G, Aydinli B, Kizrak Y, Eren S, Yilmaz S. Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: comparison with T2-weighted MR cholangiography. Eur Radiol. 2013;23:2713–2722. doi: 10.1007/s00330-013-2880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito K, Siegelman ES, Stolpen AH, Mitchell DG. MR imaging of complications after liver transplantation. AJR Am J Roentgenol. 2000;175:1145–1149. doi: 10.2214/ajr.175.4.1751145. [DOI] [PubMed] [Google Scholar]
- 37.Campbell WL, Foster RG, Miller WJ, Lecky JW, Zajko AB, Lee KY. Changes in extrahepatic bile duct caliber in liver transplant recipients without evidence of biliary obstruction. AJR Am J Roentgenol. 1992;158:997–1000. doi: 10.2214/ajr.158.5.1566706. [DOI] [PubMed] [Google Scholar]
- 38.Holt AP, Thorburn D, Mirza D, Gunson B, Wong T, Haydon G. A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation. 2007;84:857–863. doi: 10.1097/01.tp.0000282805.33658.ce. [DOI] [PubMed] [Google Scholar]
- 39.Pavone P, Laghi A, Catalano C, Broglia L, Panebianco V, Messina A, Salvatori FM, Passariello R. MR cholangiography in the examination of patients with biliary-enteric anastomoses. AJR Am J Roentgenol. 1997;169:807–811. doi: 10.2214/ajr.169.3.9275901. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y, Yamashita Y, Arakawa A, Namimoto T, Mitsuzaki K, Abe Y, Katahira K, Takahashi M. Pancreaticobiliary ductal system: value of half-Fourier rapid acquisition with relaxation enhancement MR cholangiopancreatography for postoperative evaluation. Radiology. 2000;215:81–88. doi: 10.1148/radiology.215.1.r00ap0281. [DOI] [PubMed] [Google Scholar]
- 41.Pecchi A, De Santis M, Gibertini MC, Tarantino G, Gerunda GE, Torricelli P, Di Benedetto F. Role of magnetic resonance imaging in the detection of anastomotic biliary strictures after liver transplantation. Transplant Proc. 2011;43:1132–1135. doi: 10.1016/j.transproceed.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708–718. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 43.Boraschi P, Braccini G, Gigoni R, Sartoni G, Neri E, Filipponi F, Mosca F, Bartolozzi C. Detection of biliary complications after orthotopic liver transplantation with MR cholangiography. Magn Reson Imaging. 2001;19:1097–1105. doi: 10.1016/s0730-725x(01)00443-x. [DOI] [PubMed] [Google Scholar]
- 44.Shaikh F, Elazzazi M, Ryan A, Semelka RC. Debris-filled biliary system: a difficult diagnosis on MRI and MRCP. Clin Imaging. 2012;36:153–155. doi: 10.1016/j.clinimag.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Gor NV, Levy RM, Ahn J, Kogan D, Dodson SF, Cohen SM. Biliary cast syndrome following liver transplantation: Predictive factors and clinical outcomes. Liver Transpl. 2008;14:1466–1472. doi: 10.1002/lt.21492. [DOI] [PubMed] [Google Scholar]
- 46.Kim YK, Kim CS, Lee JM, Ko SW, Chung GH, Lee SO, Han YM, Lee SY. Value of adding T1-weighted image to MR cholangiopancreatography for detecting intrahepatic biliary stones. AJR Am J Roentgenol. 2006;187:W267–W274. doi: 10.2214/AJR.05.0266. [DOI] [PubMed] [Google Scholar]
- 47.Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547–557. doi: 10.7326/0003-4819-139-7-200310070-00006. [DOI] [PubMed] [Google Scholar]
- 48.Boraschi P, Donati F, Gigoni R, Salemi S, Urbani L, Filipponi F, Falaschi F, Bartolozzi C. Complications after liver transplantation: evaluation with magnetic resonance imaging, magnetic resonance cholangiography, and 3-dimensional contrast-enhanced magnetic resonance angiography in a single session. Can Assoc Radiol J. 2008;59:259–263. [PubMed] [Google Scholar]
- 49.Laghi A, Pavone P, Catalano C, Rossi M, Panebianco V, Alfani D, Passariello R. MR cholangiography of late biliary complications after liver transplantation. AJR Am J Roentgenol. 1999;172:1541–1546. doi: 10.2214/ajr.172.6.10350286. [DOI] [PubMed] [Google Scholar]
- 50.Cereser L, Girometti R, Como G, Molinari C, Toniutto P, Bitetto D, Zuiani C, Bazzocchi M. Impact of magnetic resonance cholangiography in managing liver-transplanted patients: preliminary results of a clinical decision-making study. Radiol Med. 2011;116:1250–1266. doi: 10.1007/s11547-011-0707-4. [DOI] [PubMed] [Google Scholar]
- 51.Kitazono MT, Qayyum A, Yeh BM, Chard PS, Ostroff JW, Coakley FV. Magnetic resonance cholangiography of biliary strictures after liver transplantation: a prospective double-blind study. J Magn Reson Imaging. 2007;25:1168–1173. doi: 10.1002/jmri.20927. [DOI] [PubMed] [Google Scholar]
- 52.Meersschaut V, Mortelé KJ, Troisi R, Van Vlierberghe H, De Vos M, Defreyne L, de Hemptinne B, Kunnen M. Value of MR cholangiography in the evaluation of postoperative biliary complications following orthotopic liver transplantation. Eur Radiol. 2000;10:1576–1581. doi: 10.1007/s003300000379. [DOI] [PubMed] [Google Scholar]
- 53.Beltrán MM, Marugán RB, Oton E, Blesa C, Nuño J. Accuracy of magnetic resonance cholangiography in the evaluation of late biliary complications after orthotopic liver transplantation. Transplant Proc. 2005;37:3924–3925. doi: 10.1016/j.transproceed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 54.Katz LH, Benjaminov O, Belinki A, Geler A, Braun M, Knizhnik M, Aizner S, Shaharabani E, Sulkes J, Shabtai E, et al. Magnetic resonance cholangiopancreatography for the accurate diagnosis of biliary complications after liver transplantation: comparison with endoscopic retrograde cholangiography and percutaneous transhepatic cholangiography - long-term follow-up. Clin Transplant. 2010;24:E163–E169. doi: 10.1111/j.1399-0012.2010.01300.x. [DOI] [PubMed] [Google Scholar]
- 55.Xu YB, Min ZG, Jiang HX, Qin SY, Hu BL. Diagnostic value of magnetic resonance cholangiopancreatography for biliary complications in orthotopic liver transplantation: a meta-analysis. Transplant Proc. 2013;45:2341–2346. doi: 10.1016/j.transproceed.2013.03.031. [DOI] [PubMed] [Google Scholar]


