Figure 4. Integrative model for the regulation of podocyte actin dynamics in health and disease.
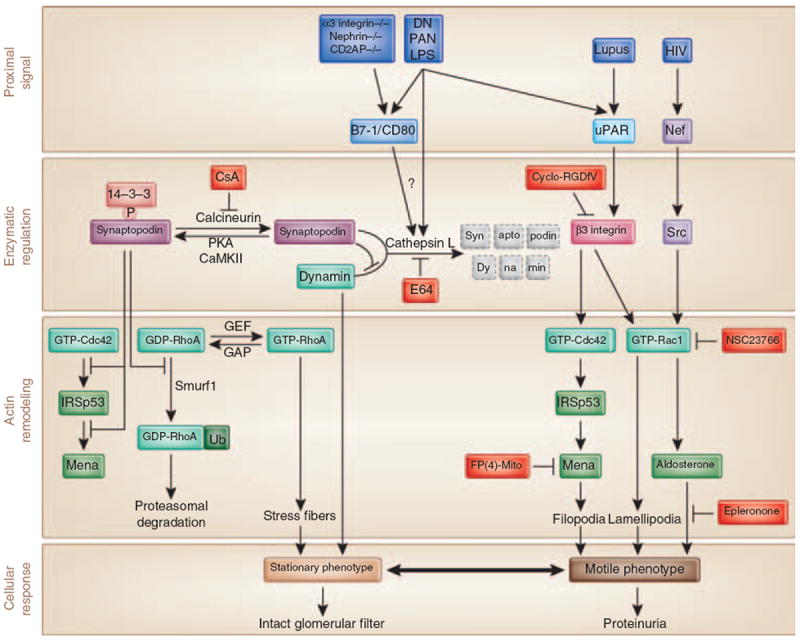
Induction of cytoplasmic cytosolic cathepsin L (CatL) enzyme is a common downstream effector in many glomerular diseases. Lipopolysaccharide (LPS) or various other proximal signals induce the expression of B7-186 and CatL58,60 in podocytes, which cause proteinuria through the increased degradation of synaptopodin57 and dynamin.60 Phosphorylation of synaptopodin by PKA or CaMKII promotes 14-3-3 binding, which protects synaptopodin against CatL-mediated cleavage, thereby stabilizing synaptopodin steady-state levels.57 Synaptopodin suppresses IRSp53–Mena-mediated filopodia by blocking the binding of Cdc42 and Mena to IRSp5396 and induces stress fibers by competitive blocking the Smurf-1-mediated ubiquitination of RhoA.26 Synaptopodin also prevents the CatL-mediated degradation of dynamin.57 Dephosphorylation of synaptopodin by calcineurin abrogates the interaction with 14-3-3. This renders the CatL cleavage sites of synaptopodin accessible and promotes the degradation of synaptopodin.57 LPS or other proximal signals can also activate Cdc42 and Rac1 through uPAR–β3-integrin signaling,42 through the loss of synaptopodin-mediated inhibition of Cdc42 signaling,96 or through Nef–Src-mediated activation of Rac1.52 As a consequence, the podocyte actin cytoskeleton shifts from a stationary to a motile phenotype, thereby causing foot process effacement and proteinuria. CsA and the CatL inhibitor E64 safeguard against proteinuria by stabilizing synaptopodin and dynamin steady-state protein levels in podocytes, FP(4)-Mito by blocking Cdcd42–IRSp53–Mena signaling,96 cycloRGDfV by blocking uPAR–β3-intergrin signaling,42 NSC23766 by blocking Rac1, and epleronone by blocking aldosterone signaling.20
