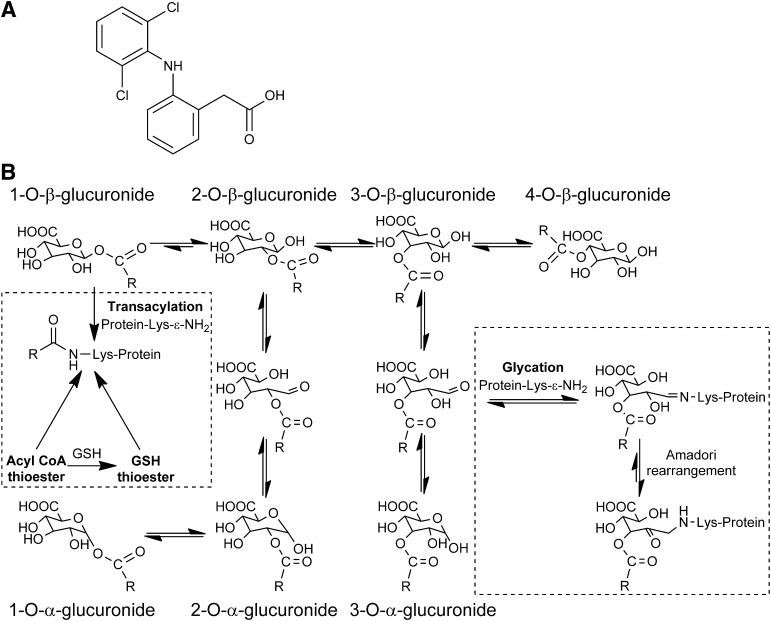
Fig. 1.
(A) Diclofenac. (B) Proposed mechanisms of protein adduction at lysine residues by reactive AG and thioester metabolites of diclofenac in vivo. Synthetic diclofenac 1-β AG acylated and/or glycated up to ten of HSA’s 59 lysines in vitro depending on the molar ratio (Table 2). Between one and six modified HSA lysines were identified in each diclofenac patient (Table 4). The acylation adducts and complete glycation adducts were found in all and three of the six patients, respectively. In vivo, only complete glycation adducts, retaining the glucuronyl and drug carboxyl residues, are unambiguously formed from AG metabolites. The acylation adducts, as discussed in the text, might also be formed in vivo from thioester metabolites of coenzyme A (CoA) and glutathione (GSH). Only the 1-α AG, produced from 2-α AG by reverse acyl migration, was seen in vitro, but in principle all of the anomers can be formed. The 1-β AG, as shown here, is the predominant acylating isomer in vitro, but involvement of the three regioisomers and their anomers cannot be excluded. Lysine N-ε-glycation by the 3-β AG is purely representative; the regioisomers involved were not identified. The extent of any Amadori rearrangements of hydroxyimine adducts (Schiff bases) of the C-3 and C-4 esters to ketoamines is unknown.