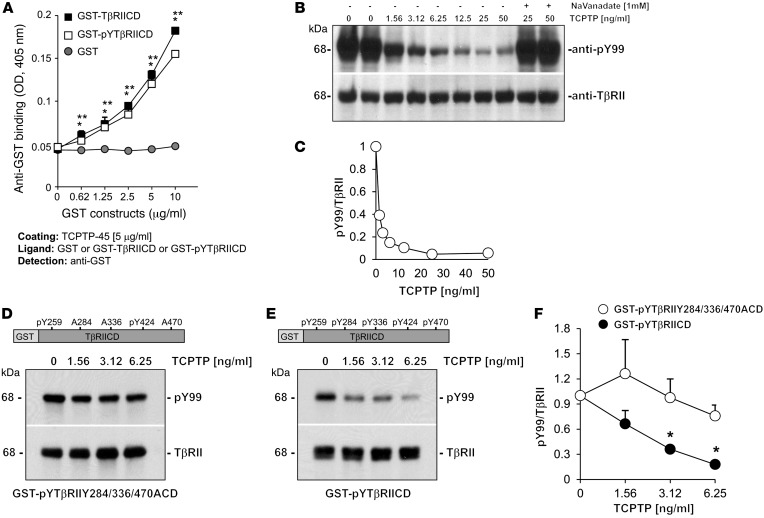
Figure 8. TCPTP directly binds and dephosphorylates the cytoplasmic tail of TβRII.
(A) Immobilized TCPTP (5 μg/ml) was incubated with GST, GST-TβRIICD, or GST-pYTβRIICD at the indicated concentrations. Bound proteins were detected with anti-GST antibodies. Shown is 1 experiment performed in triplicate, representative of 2 independent experiments performed with similar results. Values are mean ± SD. *P ≤ 0.05 vs. GST-pYTβRIICD; **P ≤ 0.05 vs. GST. (B) GST-pYTβRIICD (~50 ng) was incubated with TCPTP-37 at the indicated concentrations, with or without the tyrosine phosphatase inhibitor sodium vanadate. After 10 minutes at 30°C, samples were analyzed by Western blot for levels of phosphorylated (anti-pY99) and total (anti-TβRII) GST-pYTβRIICD. (C) pY99 and TβRII bands were quantified by densitometry. Values represent pY99/TβRII ratio relative to samples incubated without TCPTP (assigned as 1). (D and E) GST-pYTβRIICD or GST-pYTβIIY284/336/470ACD (~50 ng) was incubated with TCPTP-37 at the indicated concentrations. After 10 minutes at 30°C, samples were analyzed by Western blot for levels of phosphorylated (anti-pY99) and total (anti-TβRII) GST-conjugated recombinant proteins. (F) pY99 and TβRII bands were quantified by densitometry. Values (mean ± SEM of 3 experiments) represent pY99/TβRII ratio relative to samples incubated without TCPTP (assigned as 1). *P ≤ 0.05 vs. 0 ng/ml.