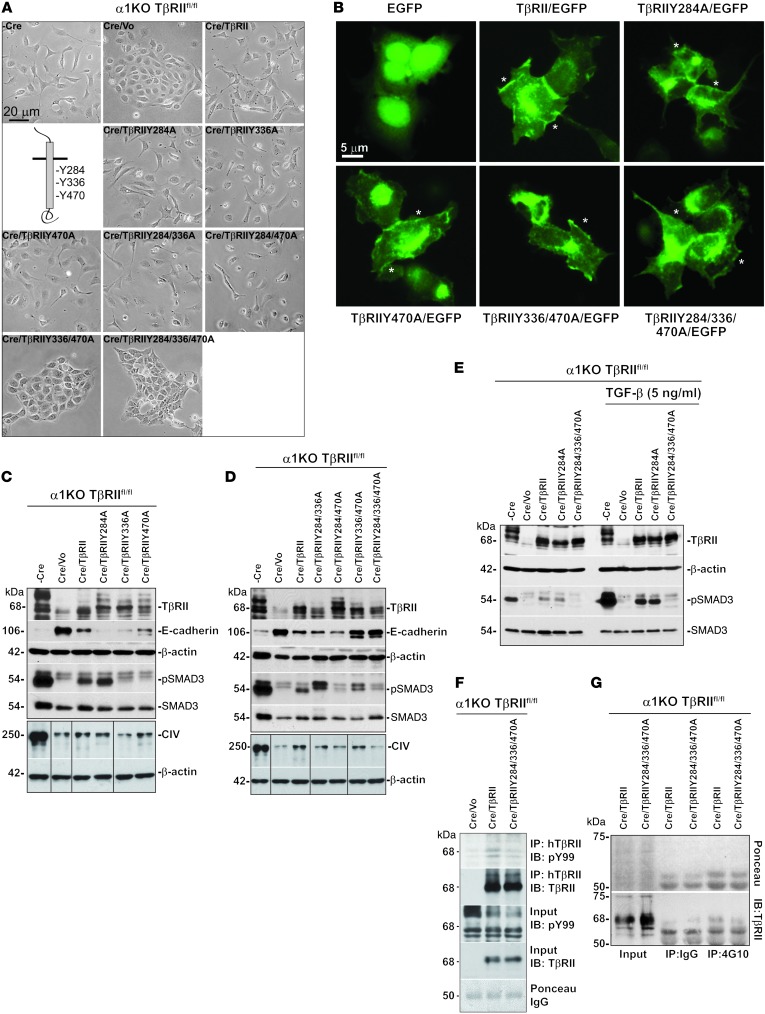
Figure 9. Y336 and Y470 in the cytoplasmic tail of TβRII regulate EMT.
(A) α1KO TβRIIfl/fl CD cells were left untreated (–Cre) or treated with adeno-Cre (Cre) to downregulate TβRII. Cells were then transfected with empty vector (Cre/Vo), WT TβRII (Cre/TβRII), or TβRII constructs mutated in 1 or more of the 3 tyrosines (as indicated), and their morphology was evaluated. (B) HEK293 cells were transiently transfected with empty pEGFP-N2 vector (EGFP), WT TβRII (TβRII/EGFP), or TβRII constructs mutated in 1 (TβRIIY284A/EGFP and TβRIIY470A/EGFP) or multiple (TβRIIY336/470A/EGFP and TβRIIY284/336/470A/EGFP) tyrosines. After 72 hours, the membrane localization (asterisks) of the various TβRII constructs was evaluated by analyzing the cells under an epifluorescence microscope. (C and D) Cell lysates (20 μg/lane) from the serum-starved CD cell populations indicated were analyzed by Western blot for levels of TβRII, pSMAD3, SMAD3, collagen IV, and E-cadherin. Lanes were run on the same gel but were noncontiguous (black lines). (E) The indicated CD cell populations were serum starved for 24 hours, then treated or not with TGF-β1 for 30 minutes. Cell lysates (20 μg/lane) were analyzed by Western blot for levels of TβRII, pSMAD3, and SMAD3. (F and G) Cell lysates (0.5 mg) from the serum-starved CD cell populations indicated were immunoprecipitated with anti-human TβRII antibodies (2 μg) (F) or with 4G10 (10 μg) or mouse IgG isotype control antibody (10 μg) (G), then analyzed by Western blot. A band corresponding to TβRII was more tyrosine phosphorylated (F) and more evident (G) in lysates of CD cells expressing WT than Y284/336/470A TβRII. Scale bars: 20 μm (A); 5 μm (B).