FIGURE 4.
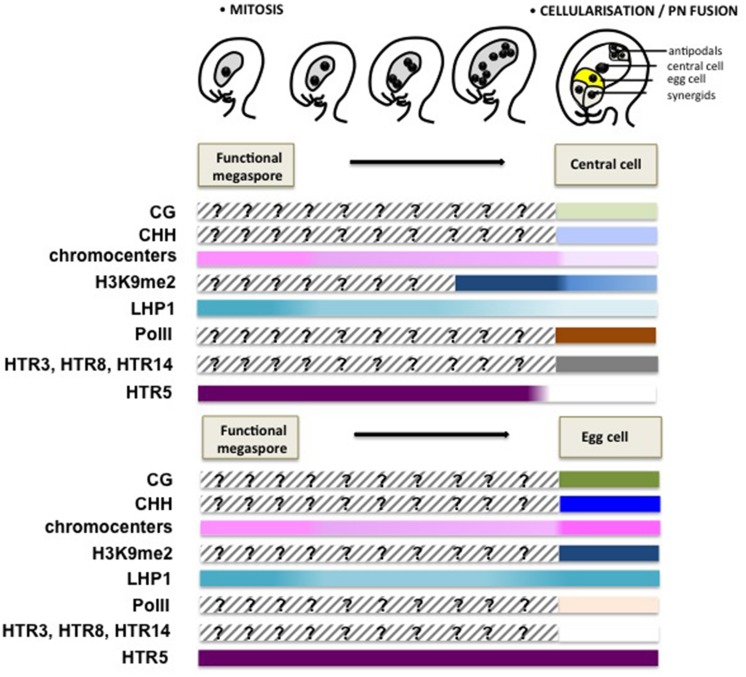
Chromatin dynamics during female gametogenesis. This scheme summarizes mostly cytogenetic and GFP reporter protein analyses suggesting large-scale chromatin dynamics events during female gametophyte development. Although genome-wide, molecular profiling of the chromatin state is currently missing, these data provide, like for Figure 3, a conceptual framework for apprehending the extent and potential significance of chromatin dynamics during this developmental stage. Following cellularization, a dimorphic chromatin landscapes are established between the egg cell and the central cell. The central cell chromatin harbors a decondensed chromatin with a low heterochromatin content, correlating with low levels of H3K9me2 and the H3K27me3 reader protein LHP1, but is enriched in active PolII (Ser2 phosphorylated PolII) allowing for active transcription (Pillot et al., 2010). The notable absence of DNA methyltransferases and the presence of the DNA glycosylase DEMETER catalyzing DNA methylation suggest a hypomethylated genome. In contrast, the egg cell harbors heterochromatin foci, though not as prominently as in somatic nuclei and high levels of H3K9me2 and LHP1, but undetectable levels of PolII, suggesting a repressed transcriptional state. Somatic histone variants are depleted from both gametes, with only HTR3, HTR8 and HTR14 retained in the central cell and HTR5 in the egg cell. The model for dynamic changes of CG and CHH methylation is speculative, and is inferred from the analysis of DNA methylation in the endosperm and embryo (Hsieh et al., 2009; Ibarra et al., 2012), as well as the differential expression of DNA methyltransferases between the central cell and egg cell (Jullien et al., 2012). The epigenetic dimorphism concerning heterochromatin content, H3K9me2 and LHP1 seems established just after cellularization.