Figure 4.
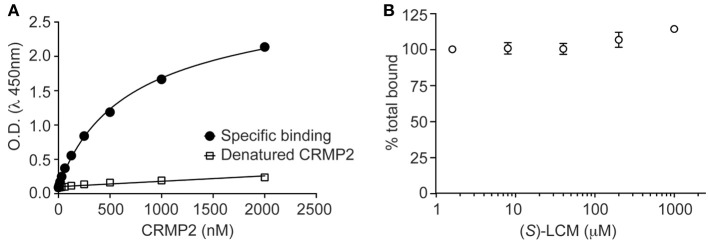
(S)-LCM does not affect tubulin-CRMP2 binding. (A) 96-well plates coated with 200 ng tubulin were incubated with increasing concentrations of recombinant CRMP2 or heat-denatured CRMP2. The Y axis displays the OD450 absorbance of the ELISA using CRMP2-specific antibodies. CRMP2 bound to tubulin with half-saturation concentration of ~607 nM. Binding of heat-denatured CRMP2 demonstrated non-saturable background binding to tubulin. (B) Competitive binding assay revealed that (S)-LCM does not abrogate the binding of CRMP2 to tubulin. For (A,B), all measurements were performed in sextuplicate and error bars indicate standard error of the mean. Most of the error bars are smaller than the symbols.
