Fig. 64.1.
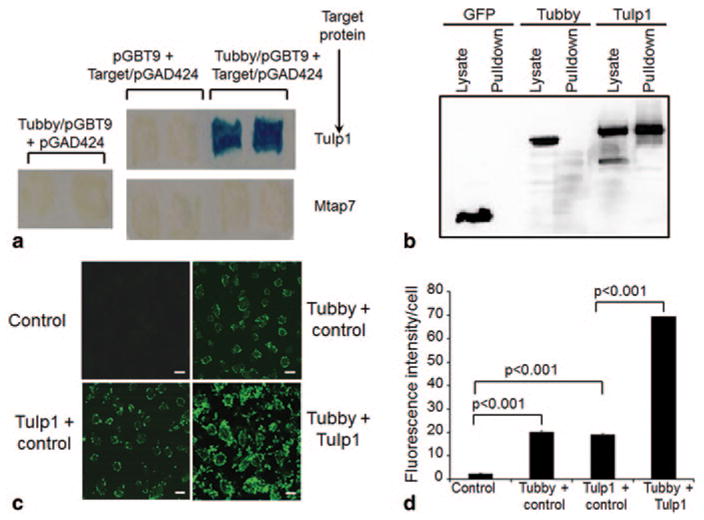
Tubby and Tulp1 interact and synergistically stimulate RPE phagocytosis. a Yeast two-hybrid assay. Tubby N-terminal domain in pGBT9 plasmid and Tulp1 in pGAD424 plasmid were co-expressed in S. cerevisiae CG-1945 stain, selected with -Leu/-Trp/-His triple dropout plates. Individual plasmids as controls were selected on -Leu/-Trp double dropout plates. Mtap7 was included as a negative control. Dropout-resistant yeast colonies were re-grown on a plate, lifted onto filter and analyzed for β-galactosidase expression with X-gal. Blue color indicated that the target protein interacts with tubby. b Protein pull-down assay. Glutathione S-transferase (GST)-tubby fusion protein was expressed, purified, incubated with cell lysates of FLAG-tagged tubby, Tulp1, or GFP (green fluorescent protein), pull-downed with glutathione resin, washed and analyzed by Western blot using anti-FLAG monoclonal antibodies. c A mixture of mGFP-labeled plasma membrane vesicles expressing tubby, Tulp1 or control was analyzed for RPE phagocytosis with human ARPE19 RPE cells for 3 h at 37 °C. Phagocytosed fluorescent signals were analyzed by confocal microscopy. Bar = 10 μm. d Relative fluorescence intensity per cell in c was quantified with > 100 cells counted per group (± SEM, n > 100, t-test). (Caberoy et al. 2010 [10] with permission from J Mol Recognit for a; Caberoy et al. 2010 [11] with permission from Exp Cell Res for b–d)
