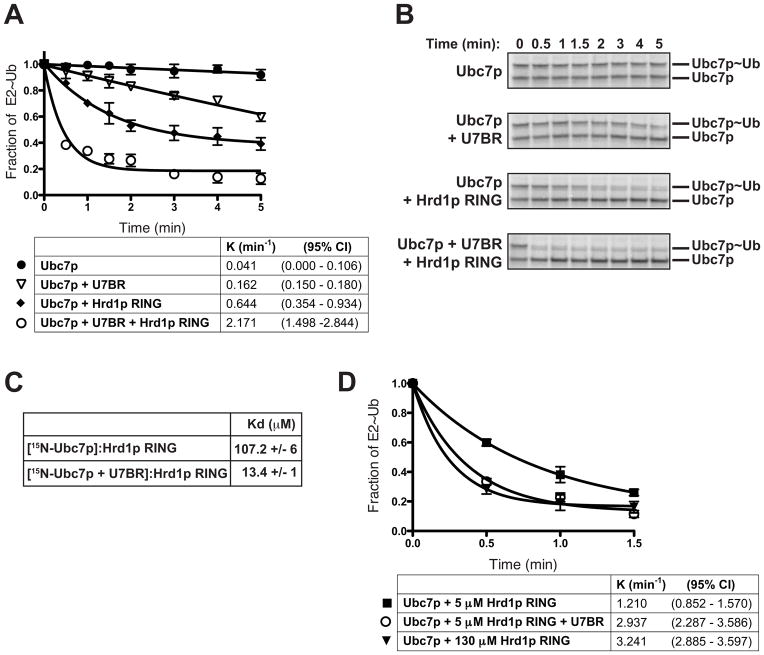
Figure 6.
The U7BR increases Ubc7p affinity for the Hrd1p RING finger domain. (A) Quantification of the rate of discharge of thioester-linked ubiquitin was analyzed as described in Figure 5A at the indicated time points in the presence of wild-type ubiquitin (80 μM) alone, or plus the U7BR (4 μM), plus the Hrd1p RING (5 μM), or plus both U7BR and RING. Data are graphed as the fraction of E2~Ub remaining at each time point from the average of three independent discharge experiments. Error bars represent the standard error; rate constant (K) and 95% confidence index (CI) are shown for each condition. (B) SDS-PAGE showing a representative experiment from (A). (C) Kd values for 15N-Ubc7p alone or bound to the U7BR for the Hrd1p RING were calculated by NMR. Error reflects the standard error from mean. Primary NMR data used to calculate these values is shown in Supplemental Figures 6A–6C; values were calculated over all peaks that shifted upon binding to the Hrd1p RING. (D) Quantification of 35S-Ubc7p discharge, performed as in (A), using the indicated Hrd1p RING concentrations. Three independent experiments were quantified and graphed as in (A). Error bars represent the standard error.