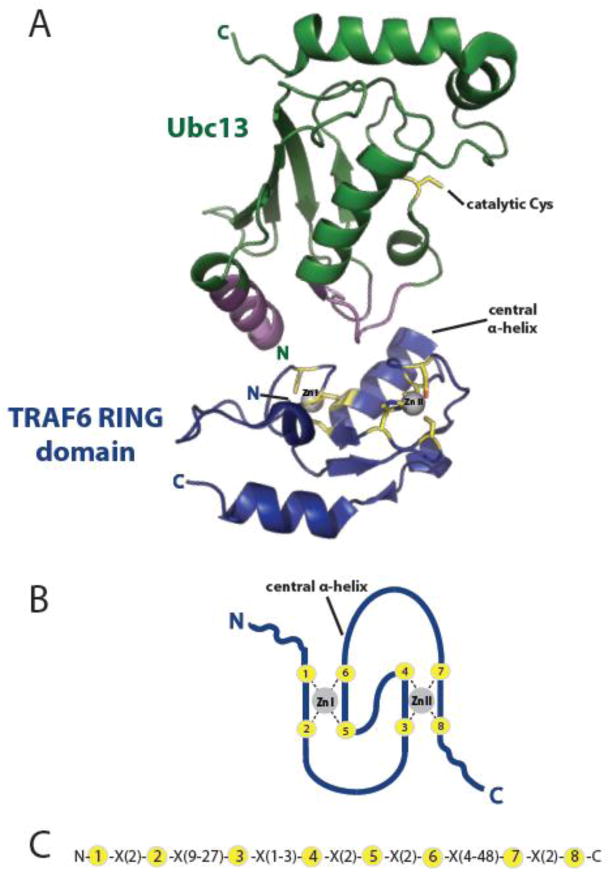
Figure 2. RING domains coordinate Zn2+ in a crossbrace arrangement that serves as a platform for E2 binding.
A) Representation of the crystal structure of the TRAF6 RING domain (blue) bound to the E2, Ubc13 (green) [89] (PDB 3HCT) highlights a stereotypical RING:E2 interaction. The catalytic Cys of Ubc13 is highlighted in yellow, while its RING domain-interacting regions are in purple. Yellow TRAF6 RING residues with sidechains shown are those that coordinate Zn2+ (C3HC3D), forming the RING crossbrace structure modeled in B). The two loops (Zn I, Zn II) and the intervening central α-helix formed by this structure together serve as a conserved platform for E2 binding. B, C) Model of the interleaved RING crossbrace structure (B) and consensus sequence (C). The eight Zn2+-coordinating residues are shown in yellow and X is any amino acid.