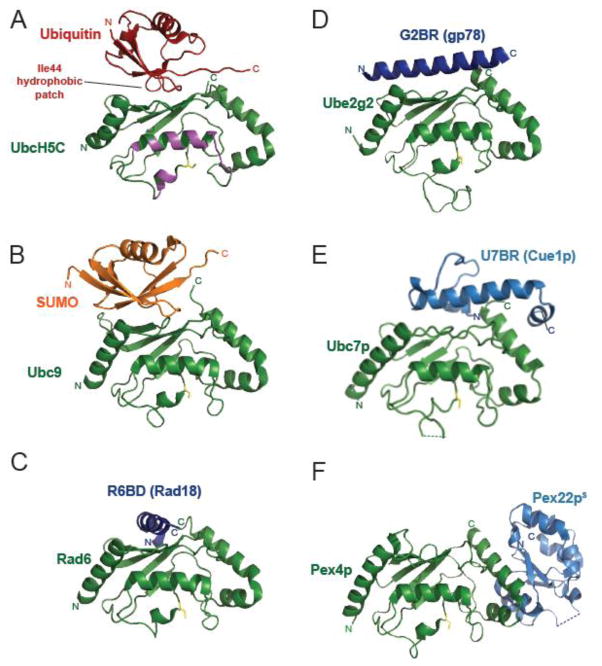
Figure 5. E2 binding domains other than the RING modulate ubiquitination by binding to the ‘backside’ of E2s.
A–F) Structures of A) ubiquitin (red, PDB 2FUH [102]); B) SUMO (orange, PDB 2UYZ [151]); or the non-RING E2 binding domains (blue) found in C) Rad18 (R6BD, PDB 2YBF [84]); D) gp78 (G2BR, PDB 3H8K [83]); E) Cue1p (U7BR, PDB 4JQU [141]); or F) Pex22p (Pex22pS, PDB 2Y9M [148]) binding to the ‘backside’ of their respective E2s (labeled and shown in green). The catalytic cysteine of each E2 is highlighted in yellow. The thioester-linked ubiquitin interaction surface is colored in magenta in panel A.