SUMMARY
Glucagon-like peptide-1 (GLP-1), an insulinotropic peptide released from the intestine after eating, is essential for normal glucose tolerance (GT). To determine whether this effect is mediated directly by GLP-1 receptors (GLP1R) on islet β-cells, we developed mice with β-cell specific knockdown of Glp1r. β-cell Glp1r knockdown mice had impaired GT after intraperitoneal (IP) glucose, and did not secrete insulin in response to IP or intravenous GLP-1. However, they had normal GT after oral glucose, a response that was impaired by a GLP1R antagonist. β-cell Glp1r knockdown mice had blunted responses to a GLP1R agonist, but intact glucose lowering with a DPP-4 inhibitor. Thus, in mice, β-cell Glp1r are required to respond to hyperglycemia and exogenous GLP-1, but other factors compensate for reduced GLP-1 action on the β-cell during meal ingestion. These results support a role for extra-islet GLP1R in oral glucose tolerance and paracrine regulation of β-cells by islet GLP-1.
INTRODUCTION
GLP-1, a peptide produced by mucosal endocrine cells in the distal intestine, is released from the gut into the circulation after nutrient ingestion. GLP-1 is generally thought to signal as a hormone, directly activating β-cell GLP1R to enhance glucose-stimulated insulin secretion, i.e. the incretin effect (Campbell and Drucker, 2013; Kieffer and Habener, 1999). In addition, GLP-1 has a broad range of actions that contribute to glucose regulation including inhibition of glucagon secretion and gastrointestinal motility, suppression of hepatic glucose production, and reduction of appetite (Barrera et al., 2011a; Campbell and Drucker, 2013). Based on these physiologic actions, the GLP1R is a logical pharmacologic target, and there are now two classes of drugs for type 2 diabetes, GLP1R agonists and inhibitors of dipeptidyl peptidase 4 (DPP-4i), that act through this receptor (Drucker and Nauck, 2006)
There are several reasons to question the conventional endocrine model proposed for GLP-1 action, a view recently expressed by several groups (D’Alessio, 2011; Holst and Deacon, 2005). First, GLP-1 circulates in relatively low concentrations and post-prandial changes in plasma levels are modest compared to other gut hormones (Baggio and Drucker, 2007; Vilsbøll et al., 2003). Second, GLP-1 is rapidly inactivated by dipeptidyl peptidase 4 resulting in a very short plasma half-life limiting availability to target cells (Deacon et al., 1995). It has been estimated that ~ 90% of secreted GLP-1 is metabolized by DPP-4 before reaching the central venous circulation (Hansen et al., 1999; Holst and Deacon, 2005). Finally, there is growing evidence that GLP-1 regulates glucose metabolism indirectly via GLP1R expressed on peripheral and central neurons (Donath and Burcelin, 2013; Vahl et al., 2007; Waget et al., 2011). This study was designed to determine whether GLP-1 mediates insulin secretion and glucose lowering as a hormone acting directly on islet β-cells.
RESULTS and DISCUSSION
β-cell GLP1R are not necessary for normal oral glucose tolerance
To address the role of β-cell GLP1R on glucose homeostasis, a Cre-loxP strategy was used to create a mouse line, Glp1rf/f, permitting tissue-specific knockdown of the Glp1r gene (Figure 1A, upper panel, and Figures S1A and S1B and Supplemental text). Mice with Glp1rf/f were crossed with animals expressing Cre recombinase ubiquitously under the control of a cytomegalovirus (CMV) promoter to create CMVcre;Glp1rΔ/Δ mice (Glp1rCMVKO) that are functionally global knockouts (Figure 1D, upper panel, and Figure S1C). The Glp1rf/f mice were also crossed with lines expressing Cre in the β-cell either under constitutive control with a rat insulin promoter (RIP) or under tamoxifen inducible regulation using a mouse insulin promoter (MIPcreER) (Kaihara et al., 2013; Wicksteed et al., 2010); (Figures S1D-S1F). To demonstrate β-cell specific disruption of Glp1r, islets were isolated from Glp1rWT, Glp1rCMVKO, RIPcre;Glp1rf/f, and tamoxifen or vehicle treated MIPcreER;Glp1rf/f mice. RNA was extracted followed by PCR of cDNA using primers that generated a product spanning the deleted exons 6 and 7 (Figure 1A, upper panel). WT mice had a transcript of 522 bp that defined the intact Glp1r gene. Islets from Glp1rCMVKO expressed exclusively a truncated cDNA of 211 bp due to deletion of the floxed portion of the Glp1r (Figure 1A, lower panel). MIPcreER;Glp1rf/f mice treated with tamoxifen, and RIPcre; Glp1rf/f mice, expressed both WT and truncated products. Islet Cre expression under the control of the CMV, RIP and MIP promoters was comparable (Figure S1H). Fidelity of Cre expression in both the RIPcre and MIPcreER lines was confirmed by crossing each with a “double reporter” Gt(ROSA)26Sortm4 (ACTB-tdTomato,-EGFP)Luo/J line (Figure 1B). RIPcre mice (Figure 1B: panel A and D), and MIPcreER mice treated with tamoxifen (Figure 1B: B and E), demonstrated robust islet-specific recombination, while MIPcreER mice treated with vehicle showed minimal recombination (Figure 1B: C and F). In contrast to the RIPcre construct, MIPcreER did not induce recombination in the hypothalamus (Figure S1G). Isolated islets, and β-cells sorted from islet cell digests, demonstrated 70–80% knockdown of Glp1r mRNA expression after tamoxifen treatment respectively (Figures S2A–S3G). Consistent with the RNA results, isolated islets from tamoxifen treated mice did not increase cytosolic cAMP (Figure 1C, upper panel), or secrete insulin (Figure 1C, lower panel), in response to the GLP1R agonist exendin-4. However, in contrast to in animals with a global deletion of Glp1r (Figure 1D, upper panel), food intake was suppressed in mice with β-cell knockdown of the Glp1r in response to GLP1R agonists (Figure 1D, lower panel), and they lost weight with chronic liraglutide treatment (Figure S2I). β-cell specific Glp1r knockdown did not affect body weight (Veh 32 ± 0.7 and Tam 31 ± 0.5 g; Figure 1E, upper panel) but caused a small, significant increase in fasting blood glucose (Veh 143 ±2 and Tam 157 ± 2.6 mg/dl, p < 0.001; Figure 1E, lower panel). Expression of proinsulin and proglucagon mRNA was similar in tamoxifen and vehicle treated MIPcreER;Glp1rf/f mice (Figure S2H).
Figure 1. Description and validation of Glp1rf/f and Cre lines.
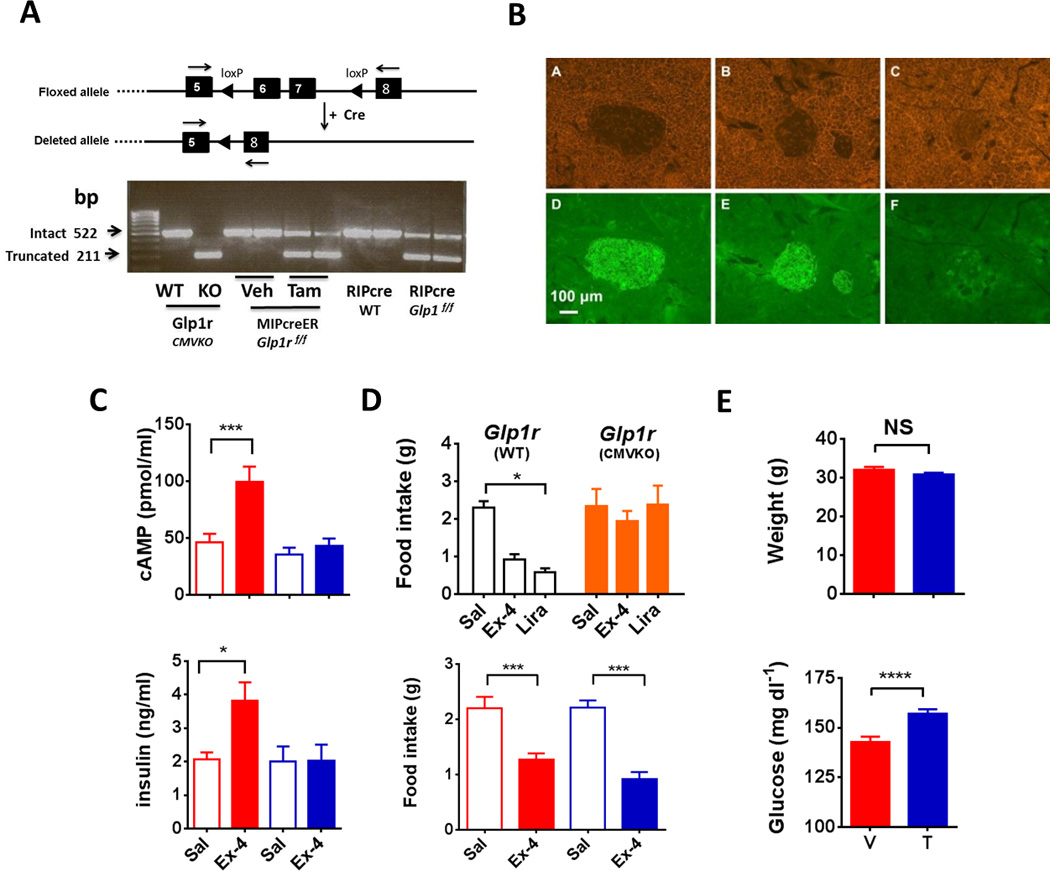
(A) Upper panel: schematic depicting the location of loxP sites inserted within Glp1r gene and the result of exons 6 and 7 deletion. Lower panel: agarose gel electrophoresis of PCR products from primers designed to generate amplicons spanning exons 6 and 7 in the Glp1r gene; the WT band is 522 bp and the truncated band 211 bp. (B) Pancreatic sections from cross of Cre lines with a “double reporter” (DR). RIPcre × DR (A, D) and MIPcreER × DR treated with tamoxifen (B, E) show diffuse EGFP in the islet under a Cy5 filter, while MIPcreER × DR given vehicle (C, F) have minimal fluorescence in β-cells. (C) Upper panel: cAMP accumulation in isolated islets (40 islets/sample, 8 mice per group, 4 separate isolations) incubated for 15 minutes in media containing IBMX with 10 nM Ex-4 or control (Vehicle red; Tamoxifen blue, *** p ≤ 0.001). (C) Lower panel: insulin concentrations in media from the islet studies described for top panel (Vehicle, red; Tamoxifen, blue, * p ≤ 0.05). (D) Upper panel: no effect of exendin-4 or liraglutide on cumulative 4 hour food intake in Glp1rCMVKO compared with Glp1rWT mice (8 per group); Lower panel: food intake in tamoxifen or vehicle-treated MIPcreER;Glp1rf/f mice (8 per group) in the 6 hours after administration of 2.5 ug Ex-4 IP or saline (*** P ≤ 0.001). (E) Upper panel: body weight in vehicle and tamoxifen treated MIPcreER;Glpr1f/f animals (78 veh and 95 tam treated mice); Lower panel: fasting glucose in vehicle and tamoxifen treated MIPcreER;Glpr1f/f animals (95 veh and 107 tam treated mice; *** P ≤ 0.001). (See also Figure S1 and Figure S2)
The effect of β-cell specific GLP1R signaling on GT was examined by comparing WT, MIPcreER;Glp1rf/f, RIPcre;Glp1rf/f, and Glp1rCMVKO mice. Compared to Glp1rWT, animals with a global deletion of the Glp1r had impaired oral GT (Figure 2A). In contrast, the glycemic response to oral glucose loading did not differ between RIPcre;Glp1rf/f and controls (Figure S3A), or between tamoxifen- and vehicle-treated MIPcreER;Glp1rf/f mice (Figure 1B), results that were repeatable in multiple separate cohorts (Figure S3C). Moreover, in response to oral glucose mice with β-cell Glp1r knockdown had similar insulin secretion (Figure 2C), comparable postprandial GLP-1 levels and diminished plasma GIP (Figure 2D) compared to controls. To further test the question of whether mealinduced GLP-1 acts directly on β-cells, glucose levels were measured in tamoxifen- and vehicle-treated MIPcreER;Glp1rf/f mice that had been trained to spontaneously ingest a fixed amount of a mixed liquid nutrients. Similar to the GTTs with gastric gavage of glucose, postprandial glucose excursions were almost identical in the two groups (Figure 2E). These findings demonstrate that during enteral glucose absorption, the setting under which GLP-1 levels increase in the circulation, β-cell GLP1R are not necessary for normal glycemia.
Figure 2. Effects of global or selective Glp1r disruption on glucose tolerance.
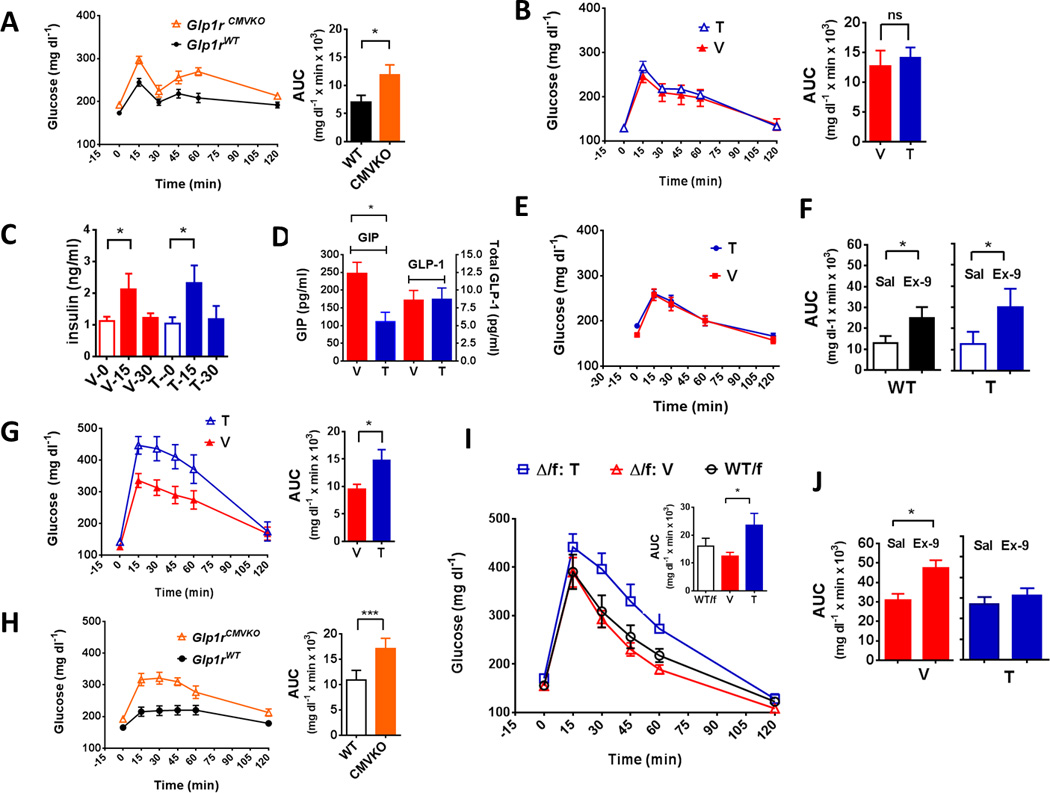
(A) Blood glucose during OGTT in Glp1rCMVKO and Glp1rWT mice with corresponding AUC. (B) Blood glucose (2.0 g/kg, 20% glucose) during OGTT in MIPcreER; Glp1rf/f mice treated with tamoxifen or vehicle (see also Figure S3C). (C) Insulin concentrations from GTT depicted in (one-tailed t test: p ≤ 0.05). (D) GIP and total GLP-1 concentrations obtained at 15 minutes following an OGTT in MIPcreER;Glp1rf/f mice treated with tamoxifen or vehicle. (E) Blood glucose following voluntarily ingested mixed liquid meal in MIPcreER;Glp1rf/f mice treated with tamoxifen (T) or vehicle (V). (F) AUC of blood glucose during OGTT in Glp1rWT/f and tamoxifen-treated MIPcreER;Glp1rf/f mice given saline or the GLP1R antagonist Ex-9 (100 µg/kg) (see Figure S3E and F for corresponding glucose curves). (G) Blood glucose (2.0 g/kg, 20% glucose) during IPGTT in MIPcreER;Glp1rf/f mice treated with tamoxifen or vehicle. (H) Blood glucose during IPGTT in Glp1rCMVKO and Glp1rWT mice. (I) IPGTT in mice with a heterozygous global Glp1r knockout, (Δ/f: Tam), β-cell-specific deletion of Glp1r (tamoxifen-treated MIPcreER;Glp1rΔ/f, Δ/f: Veh) and a full complement of Glp1r (WT; Glp1WT/f). (J) AUC of blood glucose following IPGTT in vehicle- and tamoxifen-treated MIPcreER;Glp1rf/f mice with and without Ex-9 (vehicle, red; tamoxifen, blue). Experiments used 7–11 mice per group; * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001. (See also Figure S3B for IPGTT results in RIPcre; Glp1rf/f mice)
To determine whether extra-islet GLP1R are important for normal oral glucose tolerance, WT mice or animals with β-cell specific knockdown of the Glp1r had oral glucose tolerance tests with and without the GLP1R antagonist Ex-9. Blockade of the Glp1r caused glucose intolerance in both WT mice and animals with β-cell Glp1r knockdown (Figure 1F, Figure S3E and S3F), implicating non-β-cell GLP1R in the incretin effect and regulation of postprandial glucose. Recent evidence suggests that GLP-1 has direct effects on islet α-cells (De Marinis et al., 2010), and we cannot rule out the possibility that worsening of glucose tolerance during acute GLP1R blockade is due to interference with glucagon suppression.
β-cell GLP1R are necessary for a normal response to IP glucose
In contrast to the results with oral glucose, β-cell Glp1r knockdown with either RIPcre (Figure S3B) or MIPcreER (Figure 2G) caused significant glucose intolerance in mice receiving IP glucose; this is similar to the response in Glp1rCMVKO animals (Figure 2H). Mice heterozygous for deletion of the GLP-1 receptor, MIPcreER;Glp1rΔ/f mice (See supplemental text) treated with vehicle had similar IP glucose tolerance to controls with a full complement of Glp1r (Figure 2I). MIPcreER;Glp1rΔ/f mice treated with tamoxifen, a more complete β-cell-specific knockdown, had impaired IP glucose tolerance. To determine whether the abnormal IPGTT was due to a lack of islet Glp1r, MIPcreER;Glp1rf/f mice had IP glucose tolerance tests with and without Ex-9. Acute blockade of the Glp1r caused glucose intolerance in vehicle treated mice but had no effect in animals with β-cell Glp1r knockdown (Figure 2J). These results support the importance of β-cell GLP1R in the correction of IP glucose-induced hyperglycemia. Consistent with these results, tamoxifen-treated MIPcreER;Glp1rf/f mice had higher glucose levels following IV glucose administration than vehicle-treated controls (Figure S3D). Since glucose administered IP or IV causes hyperglycemia but does not affect the release of gastrointestinal hormones or the neural activation that contribute to insulin secretion after meals (Thorens, 2011), these results suggest that β-cell GLP1R are needed for normal β-cell sensitivity to hyperglycemia, independent of acute changes in circulating GLP-1.
β-cell specific knockdown of Glp1r eliminates the insulin responses to IV and IP GLP-1
To analyze the role of exogenous GLP-1 on glucose tolerance in the absence of β-cell GLP1R, tamoxifen- and vehicle-treated MIPcreER;Glp1rf/f were given IP or IV GLP-1 during an IP glucose tolerance test. Vehicle-treated mice had substantial improvement in IP glucose tolerance when given parenteral GLP-1, and this was associated with a significant increase in insulin secretion (Figures 3A–C). In contrast, the tamoxifen-treated animals had a modest reduction of glycemia when given IP GLP-1, but no increase in plasma insulin. This muted effect on glycemia is presumably the result of insulin-independent actions of GLP-1. In response to IV GLP-1, there was no effect on plasma glucose or insulin in tamoxifen-treated MIPcreER;Glp1rf/f mice (Figures 3D–3F). Similar to MIPcreER;Glp1rf/f mice, mice heterozygous for deletion of the GLP-1 receptor, MIPcreER;Glp1rΔ/f, and treated with tamoxifen, had no response to IV GLP-1. (Figures 2G and 2F). Taken together with the results of IP GLP-1 administration, the lack of an insulin response to IV GLP-1 in the setting of a robust response in vehicle-treated controls, confirms a reduction of β-cell Glp1r in tamoxifen-treated MIPcreER Glp1rf/f mice to a degree that eliminates detectable effects in vivo. Moreover, the differential effects of IP and IV GLP-1, with a partial glucose response to the former but complete absence glucose lowering with the latter, suggests that the GLP-1 system is compartmentalized, with some gluco-regulatory GLP1R sequestered from peptide in the circulation but available to peptide in the peritoneal cavity.
Figure 3. Effects of GLP-1 administration on glucose tolerance in MIPcreER;Glp1rf/f mice.
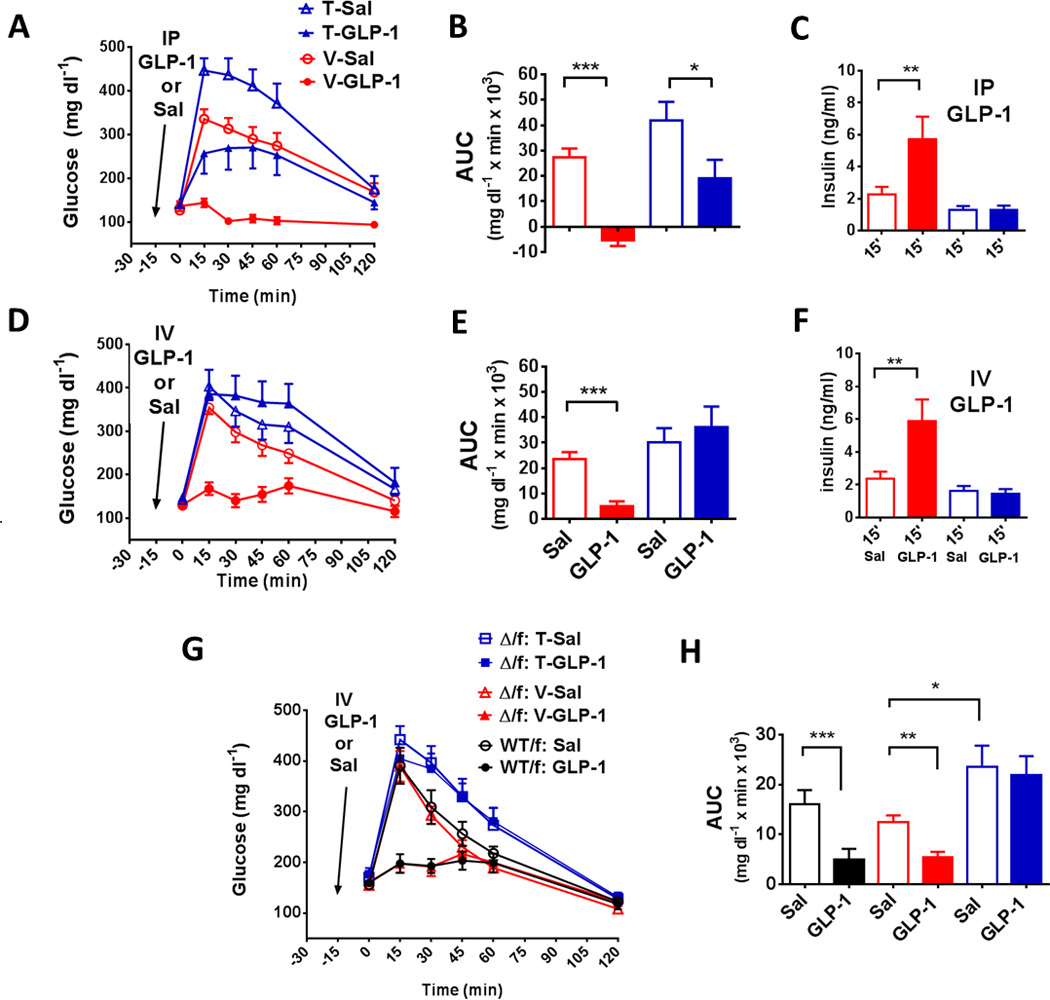
(A) Blood glucose (2.0 g/kg, 20% glucose) during IPGTT in tamoxifen- and vehicle-treated MIPcreER;Glp1rf/f mice given IP saline or GLP-1 (10 ug) 15 minutes prior to glucose injection. (B) AUC of glucose, and (C) insulin concentrations at 15 minutes, from GTTs depicted in panel A. (D) Blood glucose (2.0 g/kg, 20% glucose) during IPGTT in tamoxifen and vehicle-treated MIPcreER;Glp1rf/f given IV saline or GLP-1 (10 ug) 15 minutes prior to glucose injection. (E) AUC of glucose, and (F) insulin concentrations at 15 minutes, from mice in panel D. (G) Blood glucose during IPGTT in mice with a heterozygous global Glp1r knockout, (Δ/f: Tam), β-cell-specific deletion of Glp1r (tamoxifentreated MIPcreER;Glp1rΔ/f, Δ/f: Veh) and a full complement of Glp1r (WT; Glp1WT/f), with or without GLP-1 (10 ug, IV) given 15 min. prior to glucose. (H) AUC of glucose tolerance depicted in panel G. Experiments used 8–12 mice per group, * p ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001).
Our studies of the physiologic role of GLP1R during oral and IP glucose challenges have similarities and differences with a recent report of glucose tolerance in global Glp1r null animals with transgenic expression of a human GLP1R construct specifically in β-cells (Lamont et al., 2012). Mice with islet rescue of the GLP1R also recovered an insulinotropic response to an exogenous GLP1R agonist, similar to the results described here. However, these animals had improved oral glucose tolerance compared to Glp1r null mice, supporting a direct effect of circulating GLP-1 on β-cells, a finding that is at odds with the normal oral glucose tolerance we have observed repeatedly in mice with β-cell specific Glp1r knockdown. This discrepancy could be explained either by non-physiologic expression of the hGLP1R construct in the rescue model or insufficient knockdown of Glp1r in our inducible Cre-loxP model. Based on significant knockdown of Glp1r expression in islets and β-cells, and the inability of GLP1R agonists in vitro and in vivo to stimulate insulin release, there do not appear to be a sufficient numbers of β-cell Glp1r to mount functional responses in tamoxifen-treated MIPcreER;Glp1rf/f animals. Moreover, the normal oral glucose tolerance in this line is very reproducible, reducing the likelihood that this is an underpowered observation.
The action of long-acting GLP-1 agonists but not DPP4i are impaired with β-cell knockdown of Glp1r
To address the mechanisms by which GLP-1 signaling contributes to diabetes therapeutics, we determined the impact of β-cell Glp1r knockdown on the response to GLP-1-based drugs. Tamoxifen and vehicle-treated MIPcreER;Glp1rf/f (Figures 4A and 4B), and RIPcre;Glp1rf/f or WT (Figures S4) were given the long-acting GLP1R agonist liraglutide 30 minutes prior to an IP glucose load. The glucose profile after liraglutide was nearly flattened in the mice retaining β-cell Glp1r. Treatment with liraglutide improved glucose tolerance in mice with β-cell Glp1r knockdown, through either RIPcre or MIPcreER, but the effect was blunted compared to the controls. Quite distinct from the response to liraglutide, administration of the DPP-4i vildagliptin lowered blood glucose equivalently in vehicle and tamoxifen-treated MIPcreER;Glp1rf/f mice challenged with either oral or IP glucose (Figures 4C–F). These findings indicate that liraglutide exerts glucose lowering actions, in part, through β-cell GLP1R. The intact glucose lowering by DPP-4 inhibition may be explained by GLP-1 effects on non-β-cell GLP1R populations, or may result from compensation by other factors that are also DPP-4 substrates.
Figure 4. Effect of β-cell-specific knockdown on responses to GLP1R agonist and DPP4i.
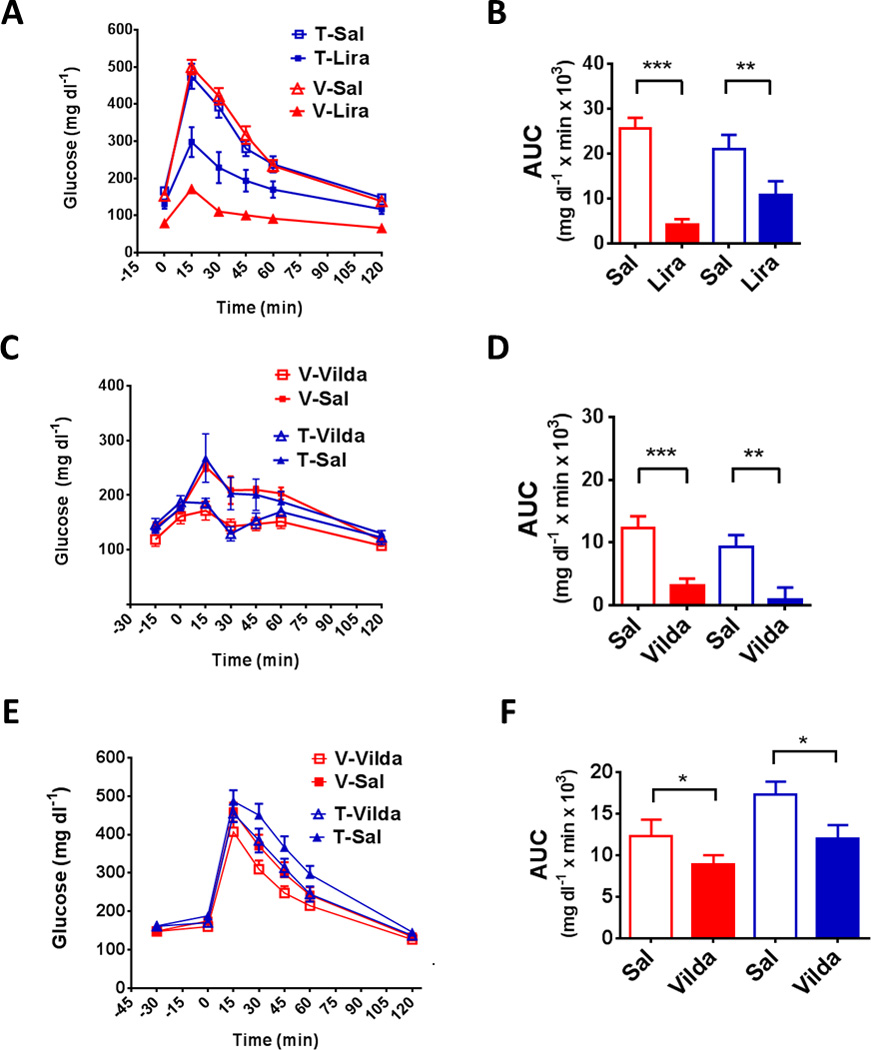
(A) Blood glucose during IPGTT in tamoxifen or vehicle-treated MIPcreER;Glp1rf/f (Tam, blue or Veh, red) mice given liraglutide (200 ug/kg) or saline 4 hours prior to glucose injection. (B) AUC from GTT in A. (C) OGTT in tamoxifen and vehicle-treated MIPcreER;Glp1rf/f mice given IP vildagliptin (150 ug) or saline (100 ul) 15 minutes before the glucose challenge. (D) AUC from GTT in C. (E) Blood glucose during IPGTT in tamoxifen- and vehicle-treated MIPcreER;Glp1rf/f mice given IP saline or vildagliptin (150 ug) 30 minutes prior to glucose injection. (F) AUC from GTT in E. Experiments used 8–16 mice per group, with * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001. (See Figure S4)
The results reported here indicate that glucose control following oral administration of carbohydrate does not require direct signaling through the β-cell Glp1r in the mouse. A major implication of these findings is that GLP-1 released into the circulation after meals does not stimulate insulin secretion through an endocrine mechanism. Rather our findings are compatible with a model in which GLP-1 acts indirectly to mediate the incretin effect, possibly through neural GLP1R. There is experimental support for neural GLP1R in the hepatic portal vein to mediate glucose tolerance (Rüttimann et al., 2009; Vahl et al., 2007) and recent evidence supports a similar mechanism in the splanchnic bed to mediate the effects DPP-4 inhibitors (Waget et al., 2011). Moreover, intra-cerebral administration of GLP-1 transiently lowers blood glucose in freely-fed rats and reduces hepatic glucose production (Barrera et al., 2011b; Burmeister et al., 2012; Sandoval et al., 2008). In this context a strong case can be made that neural GLP1R are the extra-β-cell receptors mediating glucose lowering in the present study. That β-cell GLP1R are not necessary in the setting of hyperglycemia induced by meals, but are needed for a normal response to parenteral glucose administration, may be explained by redundancy and overlap of insulinotropic signals initiated by glucose ingestion and absorption.
Our data indicate that β-cell GLP1R are necessary for the normal clearance of IP and IV glucose loads and for the insulinotropic response to exogenous GLP1R agonists. While these are experimental manipulations, a case can be made that the results have both physiologic and pharmacologic relevance. The relative responses of our knockdown and control mice to IP and IV glucose is consistent with previous work indicating that GLP-1 signaling is essential for β-cells to maintain glucose competence, or sensitivity to changes in ambient glycemia (Flamez et al., 1998; Holz et al., 1993). This effect has also been demonstrated in humans whereby Ex-9 reduces glucose-stimulated insulin release during fasting when plasma GLP-1 is low and unchanging (Salehi et al., 2010; Schirra et al., 1998). Important in this context is recent work suggesting that GLP-1 produced by α-cells in the pancreatic islet is important for local regulation of insulin secretion (Ellingsgaard et al., 2011; Kilimnik et al.; Nie et al., 2000). Our results are compatible with this model of paracrine actions of α-cell GLP-1 on β-cell GLP1R that enhance glucose-stimulated insulin release.
On the basis of the studies reported here, we conclude that the incretin role of GLP-1 cannot be explained by an endocrine mechanism of action, and that extra-islet GLP1R pathways must be invoked to explain the GLP-1 contribution to the incretin effect (See Graphical Abstract). Direct signaling through GLP1R is necessary for clearance or IV and IP glucose induced hyperglycemia, possibly by promoting glucose competence, and it is plausible that this is mediated by the actions of locally produced GLP-1. However, the role of circulating GLP-1 acting on β-cell receptors is limited to circumstances where plasma GLP-1 is substantially elevated, pharmacologically or otherwise, in a sustained manner. These findings suggest distinct levels of β-cell regulation by GLP-1 with a component of direct, paracrine or neuracrine action, and indirect mediation by extra-islet GLP1R. Moreover, our findings suggest that long-acting GLP1R agonists co-opt the paracrine system, and DPP-4 inhibitors act through non-β-cell GLP1R, to exert their anti-diabetic actions.
EXPERIMENTAL PROCEDURES
Reagents
GLP-1[7–36NH2] (21st Century Biochemicals, Marlboro, MA), vildagliptin, liraglutide, exendin-4 (Amylin, La Jolla, CA) and exendin-(9–39) (Ex-9) (21st Century Biochemicals) were reconstituted in saline containing 0.1% (w/v) bovine serum albumin, aliquotted and stored at −20 °C. Liraglutide was kindly provided by Dr. Lotte Bjerre-Nielsen, Novo Nordisk.
Animal husbandry and glucose tolerance tests
Mice were housed in a temperature-controlled room under a 12 h light-dark cycle (lights on 0600–1800 h), and fed standard chow with water available ad libitum. Oral and IP GTT’s, unless otherwise stated, were performed using 2.5g /kg, 44% glucose and 2.5 g/kg, 25% glucose, respectively, by standard methods; blood was sampled from the tail vein. Mixed nutrient OGTT was performed by training mice to consume 0.5 ml of Ensure (see Supplemental text). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Transgenic lines
The MIPcreER line generation has been previously described (Kaihara et al., 2013; Wicksteed et al., 2010) with more characterization in the Supplemental Information (see also Figure S2 and Figure S2 legend). The targeting strategy and validation of the Glp1rf/f line and the global knockout (Glp1rCMVKO) is described in Supplemental text (see also Figure S1 legend). β-cell specific Glp1r knockdowns Glp1rf/f mice were generated by crossing with either RIPcre (Jackson Labs, Tg(Ins2-cre)25Mgn, stock number 003573) or MIPcreER lines as described in the Supplemental text. A secondary MIPcreER;Glp1rΔ/f mouse line was generated following the breeding strategy of Feil (Feil et al., 2009) Supplemental Information). The MIPcreER crosses were treated at approximately 2 months of age with 1 mg IP of tamoxifen (free base, Sigma, T5648) in ethanol/sunflower oil (Feil et al., 2009) for 5 days, and experiments were started 4 weeks later. Lack of response to IV GLP-1 during an IPGTT was used to test for effective β-cell knockdown.
Food intake studies
To test the anorectic effects of exendin-4 or liraglutide, animals were placed in cages with fresh bedding, and chow removed 4 h before lights off. Mice were injected IP at the beginning of the dark phase and food intake measured after 1, 2, 4, 6 and 24 h. To assess chronic effects of liraglutide on body weight, mice were given IP liraglutide (1.0 mg/kg, SQ/day) for 14 days with food and body weight measured daily.
RNA extraction and real-time PCR
For RNA extraction, samples were processed using an RNA mini kit (QIAGEN Inc, Valencia, CA) for whole tissues, the RNA aqueous mini kit (Ambion/Life Technologies, Grand Island, NY) for mouse islets, and the RNA aqueous micro kit (Ambion) for sorted cells. cDNAs were synthesized with SuperScript® III First-Strand Synthesis kit (Invitrogen, Life Technologies). PCR primers were: β-actin, 4352341E; Glp1r KO, Mm00445292.m1; Glp1r intact, Mm0045290.m1; Gipr Mm01316344, proglucagon, Mm00801712.m1; and Ins1, Mm01259683.m1. Messenger RNA expression was calculated from the CT of target genes and β-actin using standard methods.
Assays
The following assays were performed: Insulin, ultra-sensitive rat/mouse insulin ELISA (Crystal Chem, Inc.), GIP, EMD ELISA (Millipore, Inc), cAMP, acetylated version of the cAMP ELISA kit (Cell Biolabs), and total GLP-1, ELISA (Meso Scale, Rockville, MD) as previously described (Jessen et al., 2012).
Islet cell isolation, cAMP stimulation and FACS
Islets were isolated by standard procedures (Carter et al., 2009). For cAMP experiments, 40 equally sized islets were incubated in HEPES balanced salt solution (HBSS) and 0.1% bovine serum albumin containing 3 mM glucose for 1 hour in 5% CO2 at 37°C, and then in HBSS containing 15mM glucose and 100mM IBMX, with or without 10 nM exendin-4 for 15 minutes. Islets were lysed and cAMP measured. For FACS analysis, islets were dissociated by standard methods at the Research Flow Cytometry Core at Cincinnati Children’s Hospital (Jayaraman, 2011).
Statistical Analysis
Data are presented as mean ± SEM. Analyses were performed using GraphPad Prism, version 5.01 (GraphPad Software, Inc., San Diego, CA). Comparisons of 2 samples were done with unpaired two-tailed t-tests. Analysis of multiple groups used one-way ANOVA with a multiple comparison test and two-way ANOVA was used to compare different treatments in two mouse strains.
Supplementary Material
HIGHLIGHTS.
β-cell Glp1r are not necessary for normal oral glucose tolerance in lean mice.
The incretin effect of GLP-1 is not mediated through an endocrine mechanism.
β-cell Glp1r are required for normal responses to hyperglycemia and GLP1R agonists.
DPP-4 inhibitors improve glucose tolerance through non-β-cell Glp1r.
ACKNOWLEDGEMENTS
We thank Yongmei Zhao, Todd Greer, Amanda Nunley, Sonia Lipp, Radhakrishna Krishna, and Dale Merz for technical support, Cristina Alarcon, University of Chicago, for assistance with mouse islet cell cultures, and Monica DeLay, Cincinnati Children’s Hospital Research Flow Cytometry Core (NIH P30 AR47363), for FACS analysis. Vildagliptin was a kind gift of Brian Burke, Novartis, and liraglutide graciously provided by Lotte Bjerre-Nielsen, Novo Nordisk. This study was funded by NIH DK57900.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
E.P.S, R.J.S, D.A.D designed the study and co-wrote the paper. E.P.S. performed most of the experiments. Z.A. designed and preformed a subset of the glucose tolerance tests. B.L. helped characterize the Glp1rCMVKO and Glp1rf/f lines. A.G.L performed islet cell perfusions, contributed to study design and assisted with writing. C.W. prepared islets and islet cell suspensions and designed the in vitro experiments. E.B.C. performed peptide assays. P.M performed pPCR assays. D.S and D.P-T assisted with data interpretation. L.H.P and N.T. created the MIPcreER mouse line, and D.A.S. contributed to development of the Glp1rf/f line.
DISCLOSURE SUMMARY
RJS: consults for Johnson & Johnson, and Roche; is on scientific advisory boards for Lilly, J & J, Zafgen and Merck; is a speaker for Lilly, Novo Nordisk, and Merck; has stock options in Zafgen; and research grants from J & J, Zafgen, Roche, and Mannkind.
DAD: consults for Lilly, Merck, Novo Nordisk, Roche, and Zealand and has research support from MannKind, Sanofi Aventis and Johnson & Johnson.
None of the other authors have any declarations.
References
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat. Rev. Endocrinol. 2011a;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J. Neurosci. 2011b;31:3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister Ma, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am. J. Physiol. Endocrinol. Metab. 2012;302:E334–E343. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol. Proced. Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio DA. What if gut hormones aren’t really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology. 2011;152:2925–2926. doi: 10.1210/en.2011-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- Donath MY, Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36(Suppl 2):S145–S148. doi: 10.2337/dcS13-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011;17:1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil S, Valtcheva N, Feil R. Inducible Cre mice. Methods Mol. Biol. 2009;530:343–363. doi: 10.1007/978-1-59745-471-1_18. [DOI] [PubMed] [Google Scholar]
- Flamez D, Breusegem A, Van, Scrocchi LA, Quartier E, Pipeleers D, Drucker DJ, Schuit F. Mouse Pancreatic -Cells Exhibit Preserved Glucose Competence After Disruption of the. Analysis. 1998;47 doi: 10.2337/diabetes.47.4.646. [DOI] [PubMed] [Google Scholar]
- Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48:612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- Holz GG, Kühtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S. Assessment of beta cell viability. Curr. Protoc. Cytom. 2011;Chapter 6(Unit 6.27) doi: 10.1002/0471142956.cy0627s55. [DOI] [PubMed] [Google Scholar]
- Jessen L, Aulinger BA, Hassel JL, Roy KJ, Smith EP, Greer TM, Woods SC, Seeley RJ, D’Alessio DA. Suppression of food intake by glucagon-like peptide-1 receptor agonists: relative potencies and role of dipeptidyl peptidase-4. Endocrinology. 2012;153:5735–5745. doi: 10.1210/en.2012-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaihara KA, Dickson LM, Jacobson DA, Tamarina N, Roe MW, Philipson LH, Wicksteed B. β-Cell-Specific Protein Kinase A Activation Enhances the Efficiency of Glucose Control by Increasing Acute-Phase Insulin Secretion. Diabetes. 2013;62:1527–1536. doi: 10.2337/db12-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr. Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets. 2:149–155. doi: 10.4161/isl.2.3.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J. Clin. Invest. 2012;122:388–402. doi: 10.1172/JCI42497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010;11:543–553. doi: 10.1016/j.cmet.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, Zambre Y, Pipeleers D, Friedman TC. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J. Clin. Invest. 2000;105:955–965. doi: 10.1172/JCI7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59:1330–1337. doi: 10.2337/db09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J. Clin. Invest. 1998;101:1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes. Obes. Metab. 2011;13(Suppl 1):82–88. doi: 10.1111/j.1463-1326.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, Castel J, Garret C, Payros G, Maida A, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152:3018–3029. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
- Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, et al. Conditional gene targeting in mouse pancreatic β-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


