Fig. 4. A functional hypothesis on Met-substituted mutants being useful in oxidative stress response.
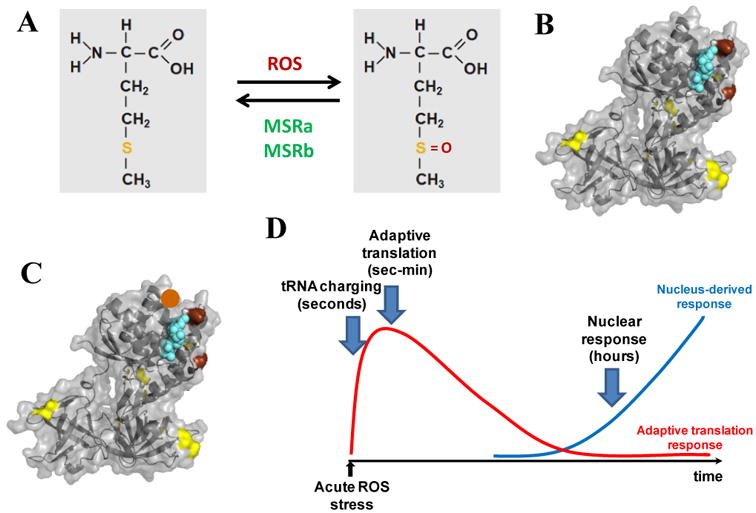
(A) Met-residues in proteins can undergo reversible oxidation by ROS and reduction by MSRa/b enzymes. (B) The E. coli EF-Tu (PDB 1efc) has two strategically positioned Met-residues (shown in brown) that protect the active site (bound GDP shown in cyan) from ROS oxidation. Other Met-residues in EF-Tu are shown in yellow. (C) A member in the Met-substituted protein library can get an extra Met-residue located at the right place (e.g. the position indicated by the brown ball) that offers extra protection. This particular mutant molecule should be more resistant against ROS inactivation. (D) tRNA misacylation-based translation may help buy time before the full engagement of the nuclear response.
