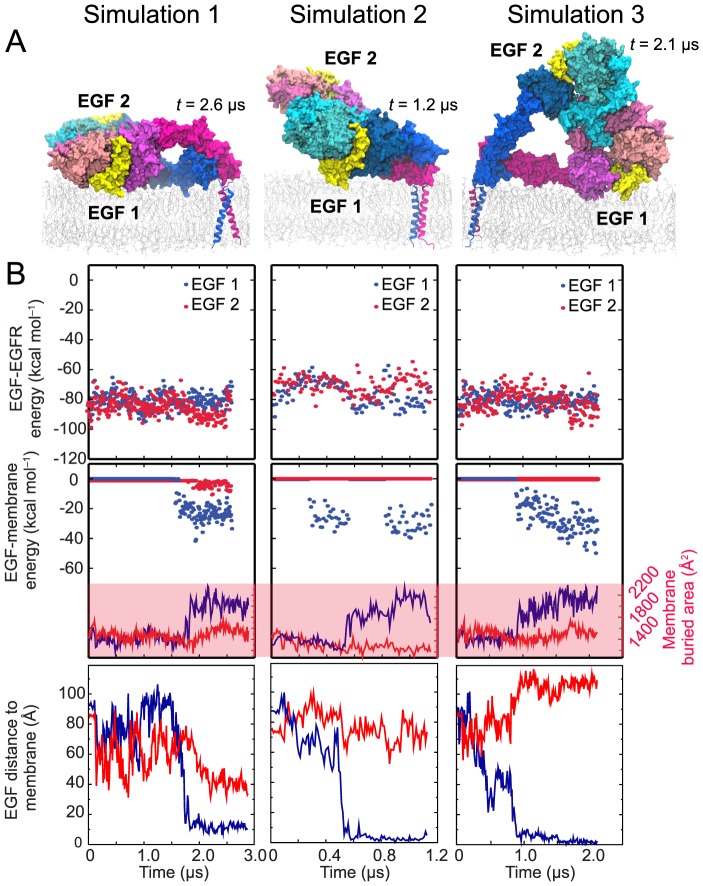
Figure 5. Ligand-membrane interaction in simulations of the two-ligand EGFR dimer.
(A) Snapshots from the endpoints of the simulations. The ectodomain dimers lie down on the membrane surface in a variety of ways; in each case, however, only one of the two ligands establishes strong interactions with the membrane. (B) The free energy of each ligand's interaction with its host receptor in a two-ligand EGFR dimer (upper panels) estimated using MM/GBVI, the strength of its interaction with the membrane bilayer (middle panels) estimated in the same way, and the distance between its N-terminus and the membrane (lower panels) in three independent simulations. In the middle panels, the surface area of each ligand buried by the membrane is plotted. As shown, the membrane-facing ligand (blue) enjoys greater binding free energy, and thus higher binding affinity, than the solvent-facing one (red) due to the additional energy conferred by the membrane interaction.