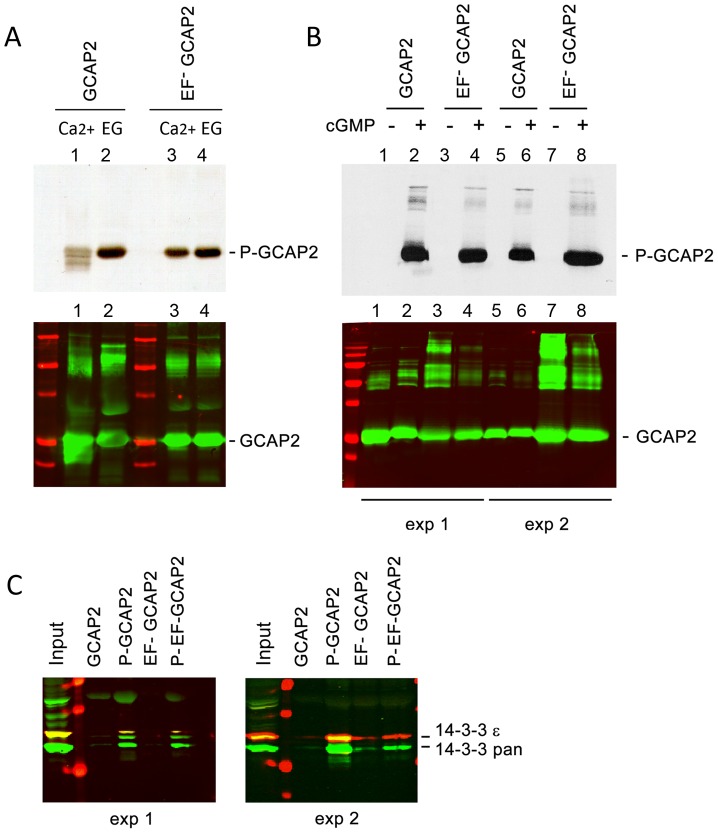
Figure 5. The protein 14-3-3 binds to recombinant GCAP2 in a phosphorylation-dependent manner.
A. In vitro, Ca2+-free bGCAP2 is phosphorylated more efficiently than Ca2+-bound bGCAP2. Upper panel shows an autoradiograph of 33P phosphorylation products from an in vitro phosphorylation reaction of recombinant wildtype bGCAP2 or bEF−GCAP2 with protein kinase G (PKG), in the presence or absence of free Ca2+. The 20 µl reaction mixture contained 8.5 µg of purified recombinant wildtype bGCAP2 or bEF−GCAP2, purified PKGIα (100 units, Calbiochem) and 3 µCi of 33P- γATP in phosphorylation reaction buffer, containing either CaCl2 or EGTA (see Methods). After incubation, reaction mixtures were resolved by 15% SDS-PAGE and transferred to a nitrocellulose membrane. Lower panel shows immunostained GCAP2. Recombinant bGCAP2 or bEF−GCAP2 protein were present to similar amounts in all reaction tubes. B. In vitro phosphorylated or mock-treated bGCAP2 and bEF−GCAP2 were generated for pull-down assays. Phosphorylation reactions were performed as above, in the presence of EGTA, except that cGMP was added to 500 µM (+ lanes) or not added (− lanes). Immunostaining of GCAP2 in the same nitrocellulose membrane shows the GCAP2 monomer at 25 kDa and upper bands corresponding to dimers and multimers of GCAP2, observed to a higher extent in the EF−GCAP2 lanes. Molecular mass (MW) markers (Precision Plus Protein Standards, BioRad) are 20, 25, 37, 50, 75, 100 and 150 kDa. Experiment shown in duplicate. C. The 14-3-3 protein isoforms bind more efficiently to phosphorylated bGCAP2 and bEF−GCAP2 than to unphosphorylated counterparts. Phosphorylated or mock- proteins were cross-liked to magnetic beads and pull-down assays were performed with whole bovine retinal extracts obtained in 1% Triton-X100. Panels show the input and bound fractions for the indicated phospho- or mock-proteins, resolved by 15% SDS-PAGE. Membrane was sequentially incubated with a pAb to 14-3-3pan (IBL International, Hamburg, Germany), a mAb to 14-3-3ε (abcam, Cambridge, UK), an IRDye 800CW Goat Anti-rabbit IgG and a IRDye 680CW Goat Anti-mouse IgG (Tebu-Bio, Offenbach, Germany). Image was acquired at the Odyssey Imaging System (LI-COR). Therefore 14-3-3pan isoforms (30 kDa) are shown in green, while 14-3-3ε (33 kDa) is shown in red. Experiment shown in duplicate.