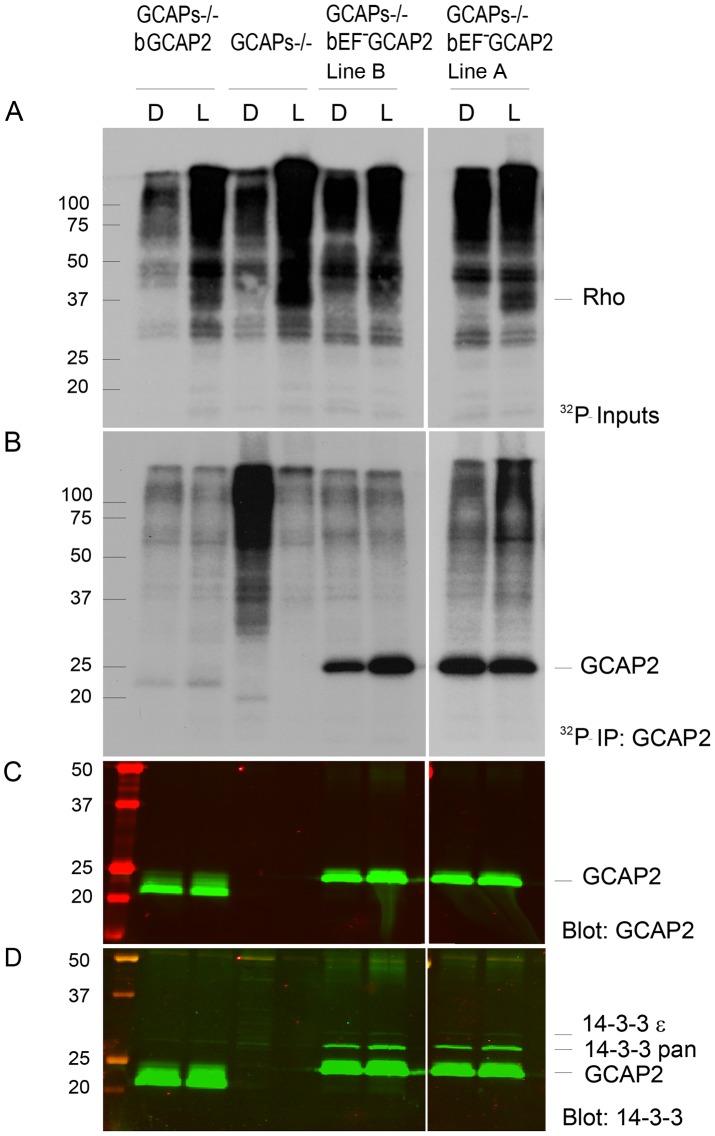
Figure 6. 32Pi metabolic labeling reveals phosphorylation of bEF−GCAP2 to a higher extent than bGCAP2 in living retinas.
A. In situ phosphorylation assay. Retinas from dark-adapted mice from the indicated phenotypes were dissected under dim red light, incubated in bicarbonate buffered Locke's solution containing 1 mCi/mL of 32P-H3PO4 for 90 min in a 5% CO2 incubator and exposed to white light for 5 min (L) or maintained in darkness (D). Retinas were homogenized in Triton X100-solubilization buffer and pre-cleared by centrifugation. Aliquots corresponding to one tenth of a retina were resolved by 15% SDS-PAGE and blotted to a nitrocellulose membrane. Phosphorylated proteins were visualized by autoradiography upon 4 h of exposure. B. GCAP2 immunoprecipitation in 32P-labeled samples. Solubilized samples corresponding to two retinas per phenotype and condition were used as inputs for GCAP2 immunoprecipitation with an anti-GCAP2 mAb. Elution fractions were resolved by 15% SDS-PAGE, blotted to a nitrocellulose membrane and visualized by autoradiography after 4 days of exposure. C. Western blot of samples in (B) using a polyclonal antibody anti-GCAP2 show that the amount of GCAP2 in the control GCAPs−/− bGCAP2 E line was comparable to that in mutant lines GCAPs−/− bEF−GCAP2 A and B. D. Immunostaining of 14-3-3 proteins in the same membrane, by using a pAb to 14-3-3pan (IBL International, Hamburg, Germany).