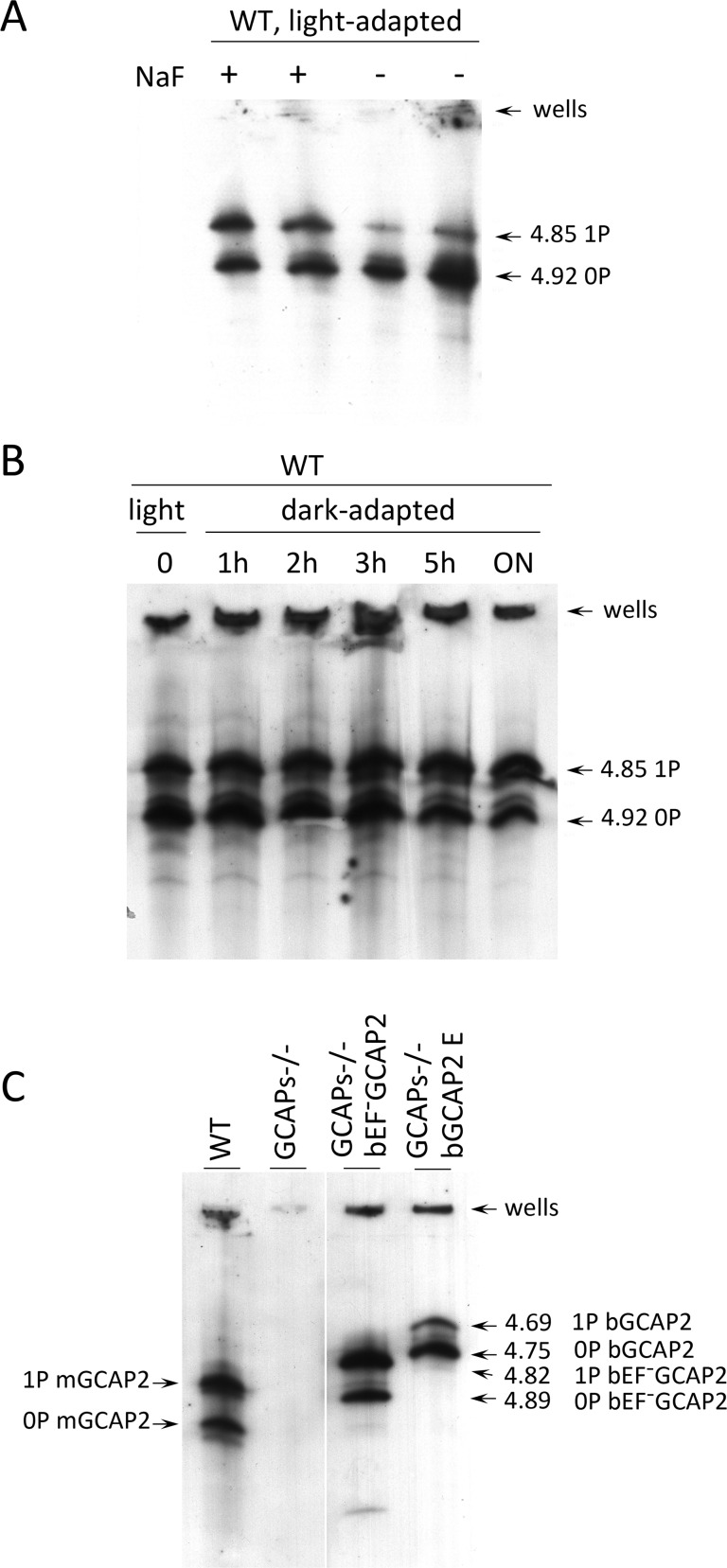
Figure 7. Analysis of GCAP2 phosphorylation by isoelectrofocusing.
A. Isoelectrofocusing (IEF) gel of light-adapted wildtype mouse retinal homogenates. Mice were light-adapted to room light. Retinas were obtained, solubilized in a saline buffer with 1% dodecyl maltoside, in the presence or absence of 50 mM NaF (phosphatase inhibitor). Samples were clarified, loaded onto an electrofocusing gel (pH range 3–8) and focused for 2 h at 23W. Proteins were transferred to a nitrocellulose membrane and incubated with an anti-GCAP2 Ab. Two prominent bands are observed at 4.92 and 4.85 isoelectric point, that correspond to unphosphorylated and monophosphorylated mGCAP2, respectively. B. The overall phosphorylation status of GCAP2 does not change significantly during a 12 h period of dark-adaptation. Mice were light-adapted to room light for 1 h, and subjected to dark-adaptation for a period of up to 14 h. Retinas were analyzed as above. C. Analysis of GCAP2 phosphorylation status in the indicated mouse lines. Transgenic bGCAP2 is phosphorylated to a lesser extent than the endogenous mGCAP2, whereas bEF−GCAP2 is phosphorylated to a much higher extent. Note that the isoelectric point of bGCAP2 differs from that of mGCAP2, and that the isoelectric point of bEF−GCAP2 (E80Q,E116Q,D158N GCAP2) is shifted versus that of bGCAP2. Results from the isoelectrofocusing gels confirm that transgenic bEF−GCAP2 is phosphorylated to a much higher extent than the control transgenic bGCAP2.