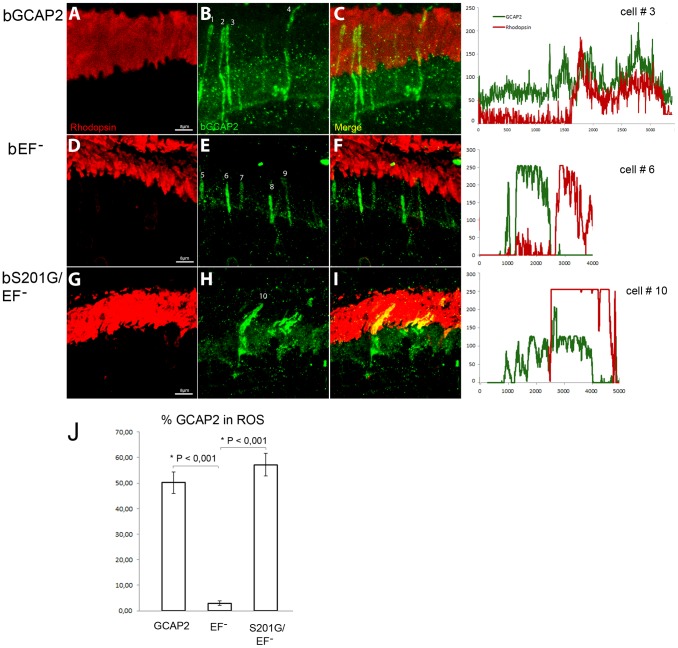
Figure 9. Mutation of Ser201 in bEF−GCAP2 precludes protein retention at the inner segment.
Wildtype bGCAP2, bEF−GCAP2 or bS201G/EF−GCAP2 were transiently expressed in the rod photoreceptor cells of GCAPs−/− mice by in vivo DNA electroporation of neonates following subretinal injection. A plasmid expressing GFP driven by the Ubiquitin C promoter was coinjected in order to identify transfected areas in the eye at postnatal day 28 (p28). Cryosections were immunostained for GCAP2 with a polyclonal GCAP2 Ab and an Alexa Fluor 555 anti-rabbit IgG (signal converted to green); and for rhodopsin with monoclonal Ab 1D4 and an Alexa Fluor 647 anti-mouse IgG (red signal). Transient expression of wildtype bGCAP2 by electroporation reproduced the reported localization pattern in the stable transgenic line, namely, its almost equal distribution between the inner and outer segments (panels A–C, profile for cell N.3). bEF−GCAP2 was mostly retained at the inner segments, being excluded from outer segments (panels D–F, and profile for cell N.6), which also reproduced the observation from the stable bEF−GCAP2 transgenic lines. In contrast, bS201G/EF−GCAP2 localized to some extent at the inner segment but mostly distributed to rod outer segments, showing a clear colocalization with rhodopsin (panels G–I, and profile from cell N.10). Panel J shows a histogram of the mean percentage of GCAP2 distribution to ROS for each phenotype and the standard error of the mean, as determined by calculating: (the intensity of GCAP2 that colocalizes with rhodopsin)/(intensity of GCAP2 that colocalizes with rhodopsin + intensity of GCAP2 at the inner segment), see Methods. (Five cells were analyzed for the WT sample, twelve cells for bEF−GCAP2 and thirteen cells for bS201G/GCAP2).