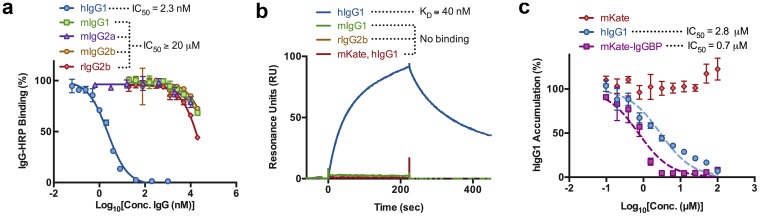
Figure 3. IgGBP fusion results in specific and high affinity binding to human IgG1 at a site overlapping the FcRn.
(a) Competition ELISA between dIgG-HRP and unlabeled IgGs binding to mKate-IgGBP coated plates. (b) Sensograms demonstrating mKate-IgGBP (100 nM) binding to immobilized hIgG1 but not mIgG1 or rIgG2b. Unmodified mKate lacks binding to immobilized hIgG1 by SPR. (c) Competition of labeled hIgG1 accumulation in MDCK hFcRn-EYFP/hβ2m cells at pH 6 by unlabeled hIgG1, mKate-IgGBP, and mKate. MDCK hFcRn-EYFP/hβ2m cells were co-incubated with 1 µM labeled hIgG1-TAMRA and increasing concentrations of unlabeled hIgG1, mKate-IgGBP, and mKate for 1 hr at 37°C and analyzed by FACS as described in the methods section. The mean fluorescent intensity (MFI) of each test protein was normalized to the average MFI of hIgG1-TAMRA accumulation in MDCK hFcRn-EYFP/hβ2m cells in the absence of unlabeled competitor and plotted as the % of hIgG1-TARMA accumulation as a function of competitor concentration. The data shown are the mean and error bars indicate s.d.