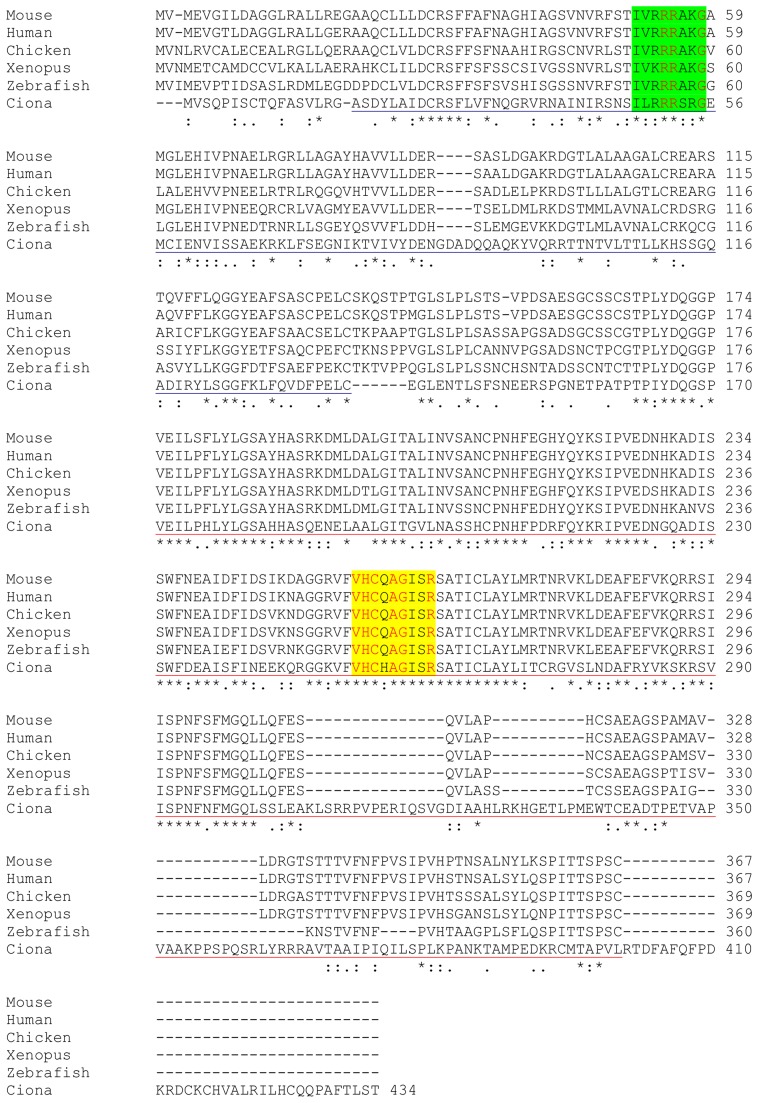
Figure 4. Multiple sequence alignment of C. intestinalis mkp1 with homologous counterparts.
Ciona sequence ci0100138796 was aligned using ClustalW with the following sequences retrieved from Uniprot database: zebrafish (Danio rerio) Q6IQU5, Xenopus laevis Q91790, chicken (Gallus gallus) F1NPN1, human P28562 and mouse P28563. The MAPK binding domain (MKB) or N-terminal domain is underlined in blue (21–136 aa in human sequence), the dual specific phosphatase domain (DSP) or C-terminal domain is underlined in red (175–367 aa in human sequence). The highly conserved catalytic motif I/VHCXAGXXR in DSP domain is highlighted in yellow, and the motif -ΨΨXRRΨXG- in the MKB domain is highlighted in green. Ψ is a hydrophobic residue and X is any residue. In red are the highly conserved positions including the catalytic cysteine (C 258 in human).