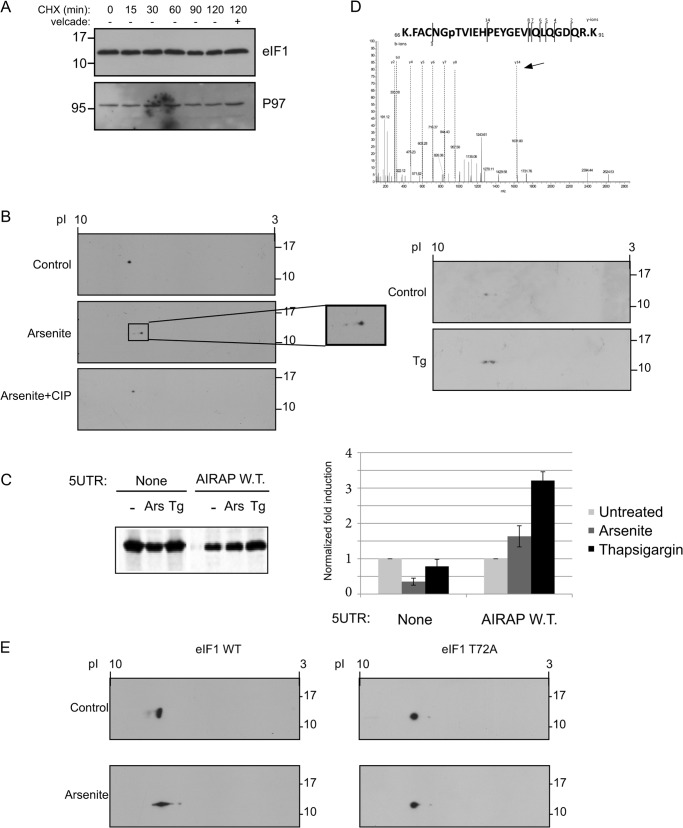
FIGURE 5.
Arsenite-induced eIF1 phosphorylation. A, eIF1 protein half-life was determined by incubating cells in the presence of 10 μg/ml cycloheximide (CHX) for the indicated time course, after which lysates were immunoblotted for eIF1 and P97 as a control for a long lived protein. Where indicated, Velcade (10 μg/ml) was added to evaluate proteasomal-dependent degradation. B, following a two-dimensional gel separation of 293T cell lysates (treated with arsenite or thapsigargin as indicated), immunoblots toward the endogenous eIF1 are presented. Where indicated (+CIP), arsenite-treated lysate was also incubated with alkaline phosphatase to evaluate if the pI shift is due to a phosphorylation event. C, autoradiogram of radiolabeled proteins obtained from 293T cells after a brief labeling pulse performed on cells that were treated as indicated. Cells were transfected with the indicated GFP reporter, subjected to a GFP IP, and content was resolved by SDS-PAGE. Untreated quantities were set as 1 for each reporter and relative quantities are shown. All quantifications were normalized to GFP mRNA transcript levels. Quantitation and statistical evaluations are present from three independent experiments. Both arsenite and thapsigargin treatments showed a statistical significant response with t test p < 0.001 upon comparing to the non-harboring 5′-UTR GFP reporter. D, fragmented MS/MS showing the only m/z fragment with a +80 shift. The presence of the y14 ion (labeled by arrow) indicates Tyr-79 is not the phosphorylation site leaving only Thr-72 as the possible site of phosphorylation. E, 24 h following the indicated eIF1 transfection, cells were treated with arsenite, and lysates were subjected to a two-dimensional gel separation analysis. Immunoblots revealing the exogenous eIF1 are presented.