FIGURE 4.
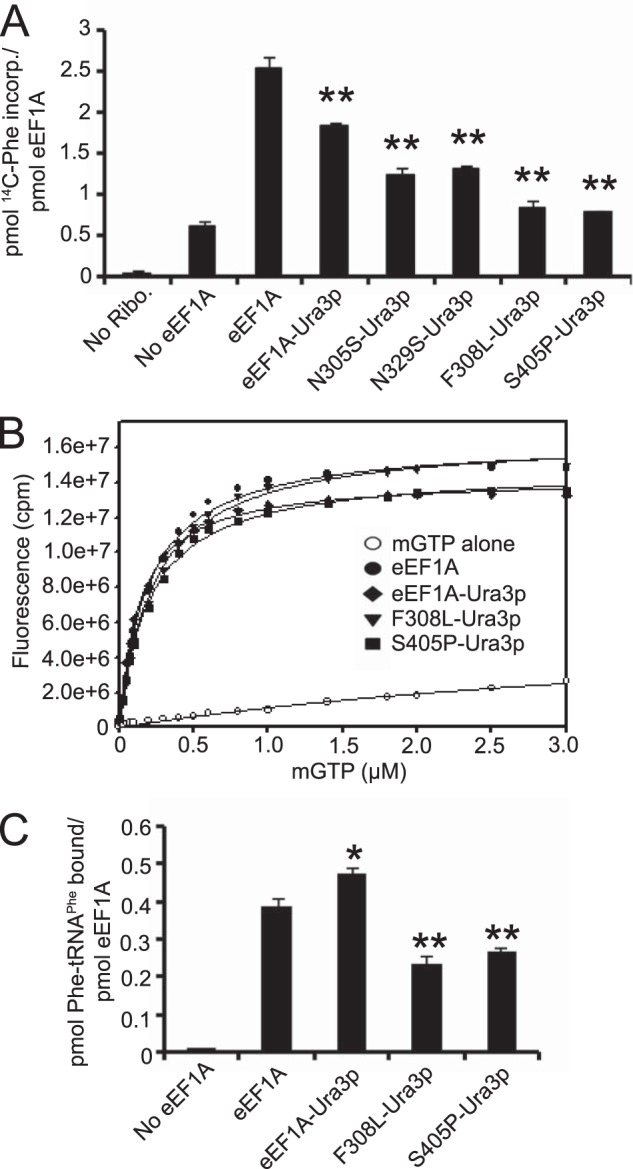
eEF1A-Ura3p mutant proteins have a defect in translation elongation. A, wild-type eEF1A, eEF1A-Ura3p, N305S-Ura3p, N329S-Ura3p, F308L-Ura3p, and S405P-Ura3p fusion proteins were purified and subjected to the poly(U)-dependent poly(Phe) synthesis assay at 37 °C for 20 min. Samples were collected on Whatman GF/C 25-mm nitrocellulose filters and the amount of radioactive phenylalanine incorporated was measured by scintillation counting. B, increasing concentrations of mant-GTP were added to 1 μm wild-type eEF1A, eEF1A-Ura3p, F308L-Ura3p, or S405P-Ura3p in binding buffer and mixed. Fluorescence was measured by FRET via excitation at 280 nm and emission at 444 nm for the mant moiety. C, purified proteins were incubated with [14C]Phe-tRNAPhe, collected on nitrocellulose filters (EMD Millipore, 0.45 μm HA) and the amount of radioactive tRNA bound was measured by scintillation counting. Significant differences relative to the wild-type protein are indicated by either one (p < 0.05) or two asterisks (p < 0.01; Student's t test).
