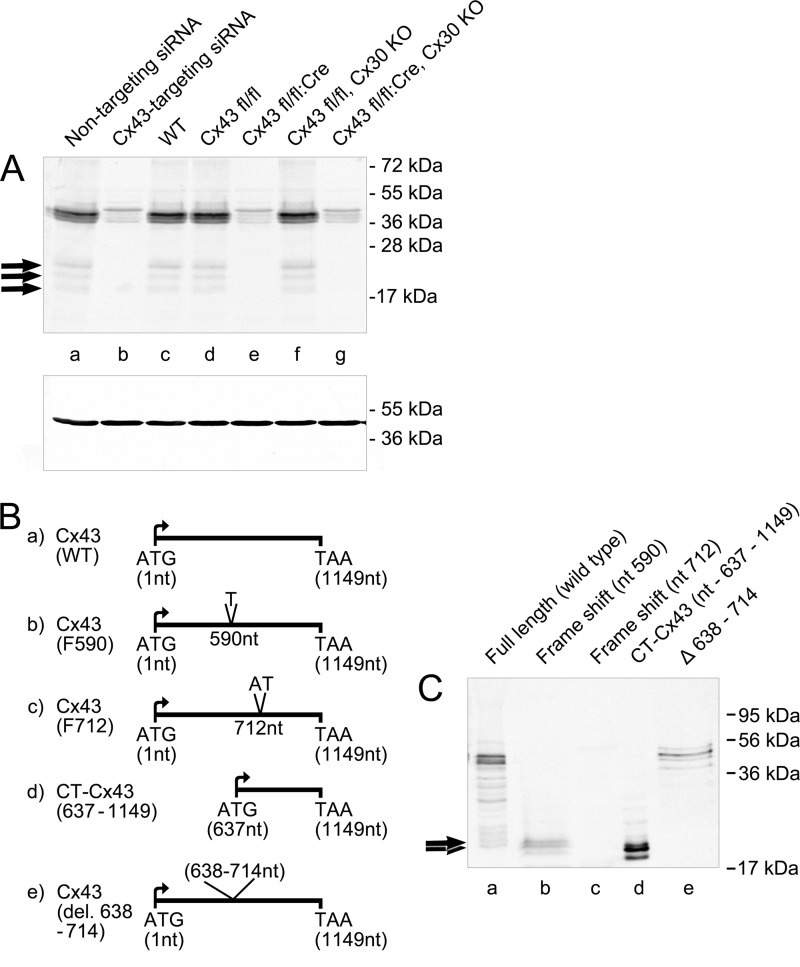
FIGURE 1.
A, existence of higher mobility protein band(s) in immunoblots of Cx43. The gel shows full-length bands and HMBs (arrows) of Cx43 from untreated (lane a) and siRNA-treated astrocytes (lane b), astrocytes from wild type mice (WT; lane c), and Cx43 homzygote floxed animals (lane d). Cx43 siRNA (lane b) Cre-activated deletion (Cx43 fl/fl:Cre; lane e) led to a loss of HMBs in astrocytes from transgenic animals, whereas full-length Cx43 isoforms were reduced by about 80%. Cx43 fl/fl animals carrying a transgene with a deleted Cx30 gene, encoding for the second major astrocytic gap junction protein, show persistent expression of Cx43-HMBs and full-length Cx43 (lane f). Deletion of both astroglial connexins (lane g) shows a pattern identical to the Cx43 fl/fl:Cre animals. Protein cleavage is not responsible for the generation of HMBs. B, schematic view of the Cx43 constructs. a, WT full-length Cx43; b, frameshift mutation at nt 590 (F590) exploited for evaluating molecular mechanisms behind the generation of endogenous CT-Cx43 domains; c, frameshift mutation at nt 712 (F712); d, construct expressing the carboxyl-terminal domain (nt 637–1149); e, construct with deletion of nt 638–714. C, Western blot of the above constructs transiently transfected into N2A cells. Lane a, WT Cx43 shows full-length Cx43 and HMBs (arrows). Lane b, frameshift F590 shows a complete loss of full-length Cx43 expression with persistent expression of HMBs (arrows). Frameshift at nt 712 (F712; lane c) displays complete loss of expression of both full-length Cx43 and HMBs. Lane d, expression of the carboxyl-terminal domains after transfection with the CT-Cx43 construct (nt 637–1149). Lane e, deletion of a sequence covering the initial carboxyl-terminal domain (nt 638–714) reveals loss of HMB expression, with persistent expression of full-length Cx43. Variability in the intensity of bands is due to differences in the expression efficiency of the different constructs.