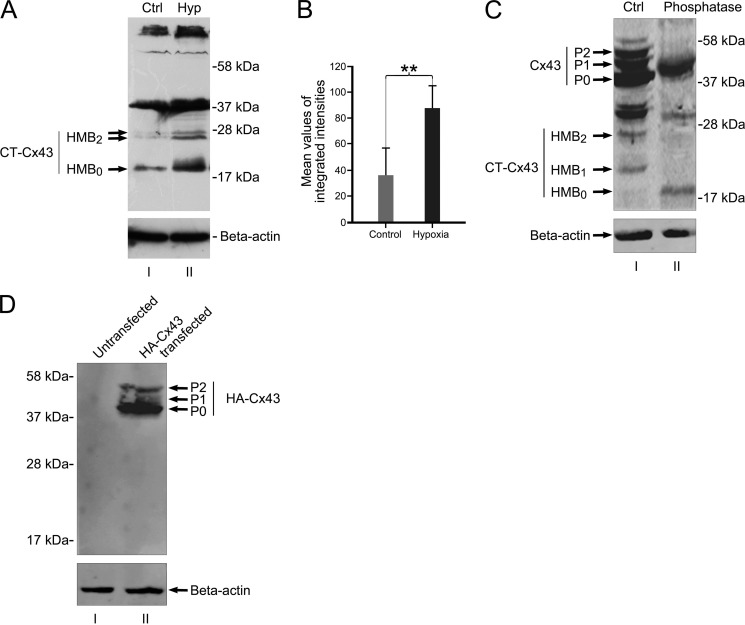
FIGURE 5.
In vivo expression of HMBs is responsive to ischemia/hypoxia. Juvenile rats respond with a significant increase of the HMB0 form after ischemia. A, HMBs of brains from untreated animals (lane I) show faint reactions at the height of HMB0 and a doublet band at HMB2, whereas the treated site reveals a robust reaction with dominance of the HMB0 form (lane II). B, mean values of the accumulated protein of all three bands indicate a significant increase (**, p < 0.01) in total protein level after ischemic/hypoxic treatment. n = 4 independent experiments. C, phosphatase treatment confirms that HMB1 and HMB2 are phosphoforms of HMB0. Lane I, protein extract from NIH3T3 cells treated with PIPES buffer, omitting phosphatase enzymes and incubated at 37 °C for 60 min (Ctrl). Lane II, NIH3T3 protein extract treated with potato acid phosphatase and incubated at 37 °C for 60 min. Phosphatase treatment resulted in the loss of P1 and P2 phosphoforms of full-length Cx43 as compared with phosphatase-omitted extract (lane I). Moreover, phosphatase treatment reveals loss of HMB1 and HMB2 and persistence of HMB0. D, Western blot of amino-terminally tagged HA-Cx43 shows no lower fragments of HA-Cx43. Lane I, untransfected NIH3T3 protein extract. Lane II, extract from HA-Cx43-transfected NIH3T3 shows full-length Cx43 isoforms (P0, P1, and P2). Further HA-Cx43 bands in the lower range are not detected by anti-HA antibodies, indicating that protein truncation of full-length Cx43 is unlikely to be responsible for the generation of high mobility bands. Error bars, S.E.